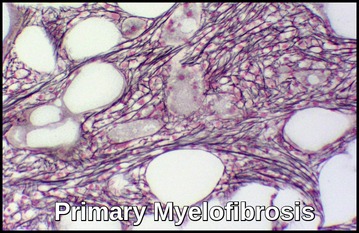
Primary Myelofibrosis
Primary Myelofibrosis (PMF) is a chronic myeloproliferative neoplasm characterized by bone marrow fibrosis, splenomegaly, and anemia with nucleated and teardrop-shaped RBCs. The spleen is often very large. Marked enlargement of the spleen and liver may result in infarction, portal hypertension, hypersplenism, plasma volume expansion, and splanchnic vein thrombosis.

Axial portal venous phase CT of the abdomen demonstrates a markedly enlarged spleen with multiple wedge-shaped and linear areas of low attenuation consistent with splenic infarctions (white arrows).
Myelofibrosis may be primary or secondary to several hematologic, malignant, and non-malignant conditions e.g. Polycythemia Vera (PRV), Essential Thrombocythemia (ET), Hairy Cell Leukemia, cancer with bone marrow metastases, osteomyelitis and TB.
Primary Myelofibrosis (PMF) is more common than secondary myelofibrosis and results from a neoplastic transformation of a multipotent bone marrow stem cell. These PMF progeny cells stimulate bone marrow fibroblasts to secrete excessive collagen.
The disease is usually idiopathic.
In this disorder, marrow fibrosis and extramedullary hematopoiesis (primarily in the liver and spleen) predominate. The fibrosis is probably reactive.
Affected individuals may not have symptoms at the time of diagnosis (asymptomatic) and may remain symptom-free for many years. Eventually, affected individuals may develop fatigue, fever, frequent infections, pale skin, night sweats and unexplained weight loss.
There is anemia with anisocytosis, poikilocytosis, and ‘teardrop’ red blood cells (dacrocytes).
The leukoerythroblastic blood picture is, as the name implies, characterized by the presence of immature forms of red and white cells in the peripheral blood. Normoblasts as well as myeloblasts (even in the absence of acute leukemia), promyelocytes, myelocytes and metamyelocytes may be present.
Serum LDH level is often elevated.
Platelet counts initially may be high, normal, or decreased; however, thrombocytopenia tends to supervene as the disorder progresses.
Bone marrow failure eventually occurs, with consequent anemia and thrombocytopenia.
Rapidly progressive, chemotherapy-incurable acute leukemia (usually M7 AML) develops in about 10% of patients.
Diagnosis:
- FBC and blood smear.
- Bone marrow examination. Bone marrow aspiration is usually dry. Because the demonstration of bone marrow fibrosis is required and fibrosis may not be uniformly distributed, a biopsy should be repeated at a different site if the first biopsy is nondiagnostic.
- Cytogenetic studies of bone marrow help exclude chronic myelogenous leukemia (CML), myelodysplastic syndrome, or other chronic myeloid disorders. However, as mentioned, these studies may be difficult to obtain due to the “dry tap” on bone marrow aspirates in over 50% of patients with primary myelofibrosis. Fluorescent in situ hybridization (FISH) studies or polymerase chain reaction (PCR) assay testing for bcr:abl may help exclude CML (this may also be performed on peripheral blood). FISH studies for abnormalities associated with myelodysplastic syndromes, such as del 7, 7q-, and 5q-, may also be helpful.
- About 50% of patients have a JAK2 mutation. Calreticulin (CALR) mutations were recently described in JAK2 and thrombopoietin receptor gene (myeloproliferative leukemia, MPL) unmutated primary myelofibrosis and essential thrombocythemia. Among 254 study patients, 147 (58%) harbored JAK2, 63 (25%) CALR and 21 (8.3%) MPL mutations; 22 (8.7%) patients were negative for all three mutations (triple-negative myelofibrosis), whereas one patient expressed both JAK2 and CALR mutations.
- U&Es.
- LFTs.
- Serum LDH and uric acid.
- Abdominal USS.
- CT scan of the abdomen.
- FDG PET/CT scan.

FDG PET/CT MIP image of a patient with myelofibrosis demonstrates diffuse FDG uptake in the bones. Note the presence of hepatosplenomegaly in the patient.
Treatment:
Historically, therapy for primary myelofibrosis was mainly supportive. Patients received transfusions as needed.
Allopurinol for gout prevention.
Thrombocytosis could be managed with Hydroxyurea and other palliative agents.
Low-risk, asymptomatic patients may be observed without intervention.
Patients with milder disease may still be treated with supportive therapies.
Ruxolitinib (Jakafi), a JAK1/JAK2 inhibitor, is the first chemotherapeutic agent to be approved by the US Food and Drug Administration (FDA) for the treatment of myelofibrosis.
Androgens, splenectomy, chemotherapy, splenic embolization and radiation therapy have been used for palliation.
Temporary use of low-dose corticosteroids may relieve symptoms.
For younger patients with advanced disease, allogeneic stem cell transplantation may be beneficial. Nonmyeloablative allogeneic stem cell transplantation has been successfully used even in older patients.
Although thalidomide and lenalidomide can alleviate anemia in myelofibrosis, their use is limited by their respective potential to cause peripheral neuropathy and myelosuppression. Pomalidomide is a second-generation thalidomide analogue with reduced toxicity and enhanced anticancer and immunological activity.
- The median survival is 5 yr from the onset, but variation is wide; some patients have a rapidly progressing disorder with short survival and some have a delay in initial diagnosis.
- Unfavorable prognostic markers include Hb < 10 g/dL, history of transfusions, leukocytosis and leukopenia, and platelet count < 100,000/μL.
- Patients in the least favorable risk group usually survive < 1 yr.
- No treatment reverses or controls the underlying process except for allogeneic stem cell transplant.
Summary:
Primary myelofibrosis (PMF) is a rare bone marrow disorder that is characterized by abnormalities in blood cell production (hematopoiesis) and scarring (formation of fibrous tissue) within the bone marrow. Bone marrow is the soft, spongy tissue that fills the center of most bones. Bone marrow contains specialized cells called hematopoietic stem cells that grow and eventually develop into one of the three main types of blood cells: red blood cells, white blood cells or platelets. In primary myelofibrosis, a change in the DNA of a single hematopoietic stem cell causes the abnormal cell to continually reproduce itself. Eventually, these abnormal cells crowd out normal, healthy cells in the marrow and, along with scarring within the marrow, disrupt the production of red and white blood cells and platelets.
The symptoms associated with primary myelofibrosis vary and are related to the abnormalities affecting blood cell production. Affected individuals may not have symptoms at the time of diagnosis (asymptomatic) and may remain symptom-free for many years. Eventually, affected individuals may develop fatigue, fever, frequent infections, pale skin, night sweats and unexplained weight loss. An enlarged (spleen) is a common finding. An enlarged liver (hepatomegaly) may also occur.
In approximately 50 percent of patients, a mutation of the JAK2 gene has been detected. The exact role this abnormal gene plays in the development of the disorder is unknown.
References:
Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res. 2007 Jun. 31(6):737-40.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013 Dec 19. 369(25):2379-90.
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013 Dec 19. 369(25):2391-405.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012 Mar 1. 366(9):799-807.
Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011 Feb 1. 29(4):392-7.
Oon, S.F., Singh, D., Tan, T.H. et al. Primary myelofibrosis: spectrum of imaging features and disease-related complications. Insights Imaging 10, 71 (2019). https://doi.org/10.1186/s13244-019-0758-y
Primary Myelofibrosis. https://rarediseases.org/rare-diseases/primary-myelofibrosis/







I have a patient of primary myelofibrosis intermediate risk with hepatosplenomegaly on ruxolitinib 25 mg bd who presented with high counts of 67 thrombocytopenia tophaceoys gout and mild pulmonary congestion likely secondary to mild LVF and AKI 1 with a edgr of 33
I reduced his ruxolitinib at 5mg bd and sent blood for flow as blood film showed blasts at 1.6%
Was the the right reduction of ruxolitinib
We are investigating the cause of HF if he has transformed to aml would you give hydroxy?
Hi,
Thank you for your message.
You have to follow the Ruxolitinib dosing guidelines. Doses may be titrated based on safety and efficacy.
If efficacy is considered insufficient and platelet and neutrophil counts are adequate, doses may be increased by a maximum of 5 mg twice daily. The starting dose should not be increased within the first four weeks of treatment and thereafter no more frequently than at 2-week intervals. For platelets 50 to less than 100 x 10(9)/L: 5 mg orally twice a day should be okay.
Duration of therapy: 6 months if no spleen reduction or symptom improvement.
BW,