Surgery and Hemostasis
What is Hemostasis?
Hemostasis is the process of how the body stops bleeding from a cut or injury. This involves forming a clot to close the hole in the blood vessel and repairing the blood vessel.
When a blood vessel is injured, platelets stick together to form a plug. Proteins, called clotting factors, interact to form a fibrin mesh to hold the platelets in place. This allows the injury to heal while preventing blood from escaping the blood vessel.
Generally, control of bleeding is achieved very quickly through the formation of a clot. In major trauma or surgery, physicians often need to help patients to achieve adequate hemostasis – in order to minimize blood loss and related injury.
However, some people are born with a bleeding disorder (congenital) that impairs their ability to achieve hemostasis. An example of this is the hereditary disorder, hemophilia.
As well, some people who have never had any bleeding problems can develop a condition that causes them to bleed, known as “acquired hemophilia”.
Blood coagulation exists to halt excessive blood loss. It is paradoxical that surgery and trauma simultaneously represent major risk factors for both hemorrhagic and thrombotic complications. Kearon and Hirsh estimated that surgery and trauma increase the baseline risk of thrombosis up to a hundred-fold, whereas patients with mild hemophilia who have never bled from stresses of everyday life may bleed vigorously following surgical procedures.
Surgery For Patients With Congenital Hemostatic Defects:
Currently, hemophiliacs can and do undergo any and all surgical procedures as dictated by their medical condition. Adequate levels of their genetically absent factor allow the operator to do whatever would be done in a similar procedure in a patient without hemophilia. A notable exception includes those hemophilia patients who have or are suspected to have an inhibitor. In this clinical scenario, it is generally not advised to perform elective surgery. Consultation with a center having experience and resources to treat these patients is in order.
Prophylaxis Against Thrombosis:
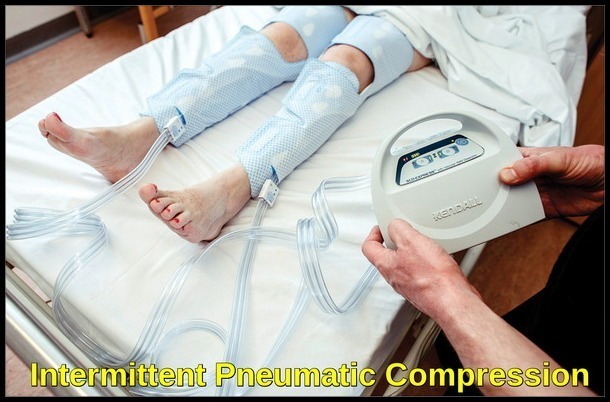
Prophylaxis against deep vein thrombosis (DVT) and pulmonary embolism in surgical patients is thoroughly reviewed in a 2012 consensus. In general, prophylaxis should be more aggressively used in hospitalized patients. Prophylaxis is safer and more effective than generally held while the toll of venous thromboembolism (VTE) without prophylaxis is usually significantly underestimated. The role of mechanical and similar adjuncts in prophylaxis against VTE verifies their use. Westrich demonstrated that intermittent pneumatic compression (IPC) devices in total knee replacement surgery were more effective than low molecular weight heparin (LMWH) and far more effective than aspirin alone in reducing both DVT and pulmonary embolism. However, they did notice only a 33% compliance rate with patients for whom IPC was prescribed. Others have demonstrated not only the effectiveness of IPC and similar devices but the additive effect of this mechanical methodology with pharmacologic prophylaxis.
There remains a reticence on the part of some surgeons citing concern of possible additional risk for hemorrhage as a result of employing VTE prophylaxis. For many indications, IPC is probably effective. However, several analyses of VTE prophylaxis in neurosurgical patients have indicated that not only is chemical prophylaxis itself well-tolerated, but when combined with IPC, it adds to the efficacy of VTE prophylaxis either without any additional risk of hemorrhage or with such a small amount of increased risk that such risk of hemorrhage is easily absorbed by the decrease in overall morbidity and mortality from VTE. Additionally, successful employment of VTE prophylaxis avoids the concern for much higher and longer dosage of therapeutic anticoagulation should a VTE occur.
Perioperative Guidelines on Antiplatelet and Anticoagulant Agents:
Direct oral anticoagulants (DOACs) have become increasingly popular for the prevention and treatment of thromboembolic disorders. However, their use can complicate perioperative management, particularly in patients undergoing surgery. The optimal timing of DOAC cessation before surgery remains unclear, and there is a lack of consensus among guidelines.
A literature search was performed using PubMed and Cochrane Library databases to identify relevant studies and guidelines published from 2010 to 2023. The search terms used were “DOACs,” “direct oral anticoagulants,” “NOACs,” “novel oral anticoagulants,” “surgery,” “perioperative management,” and “duration of holding.” Only English-language articles were included.
Results:
Several studies have investigated the optimal duration of DOAC cessation before surgery. A systematic review and meta-analysis of 11 studies involving 4,596 patients found that a DOAC interruption period of 2-4 days was associated with a lower risk of bleeding compared to shorter or longer interruption periods. Another systematic review and meta-analysis of 23 studies involving 16,834 patients found that a DOAC interruption period of 3-5 days was associated with a lower risk of bleeding compared to shorter or longer interruption periods.
Guidelines from various organizations have also provided recommendations on the duration of holding DOACs before surgery. The American College of Chest Physicians (ACCP) recommends holding DOACs for at least 48 hours before surgery for low-risk procedures and up to 7 days for high-risk procedures. The European Society of Cardiology (ESC) recommends holding DOACs for at least 24 hours before low-risk procedures and up to 4 days for high-risk procedures. The International Society on Thrombosis and Haemostasis (ISTH) recommends holding DOACs for at least 24 hours before low-risk procedures and up to 5 days for high-risk procedures.
Conclusion:
The optimal duration of holding DOACs before surgery remains a topic of debate, with varying recommendations from different guidelines. However, the available evidence suggests that a DOAC interruption period of 2-5 days is associated with a lower risk of bleeding compared to shorter or longer interruption periods. Clinicians should consider the individual patient’s bleeding and thrombotic risk, as well as the type and urgency of the planned procedure when deciding on the appropriate duration of DOAC cessation.
Vitamin K antagonists should be stopped 3 to 5 days before surgery. Preoperative laboratory testing is recommended. Bridging therapy does not decrease the perioperative thromboembolic risk and might increase the perioperative bleeding risk. In patients on direct-acting oral anticoagulants (DOAC), a discontinuation interval of 24 and 48 h in those scheduled for surgery with low and high bleeding risk, respectively, has been shown to be saved. Several guidelines for regional anesthesia recommend a conservative interruption interval of 72 h for DOACs before neuraxial anesthesia. Finally, aspirin is commonly continued in the perioperative period, whereas potent P2Y12 receptor inhibitors (clopidogrel, ticlopidine, ticagrelor, prasugrel, and cangrelor) should be stopped, drug-specifically, 3 to 7 days before surgery.
Bridging Anticoagulation:
Patients who are on long-term anticoagulant therapy and require temporary interruption of their medication for a surgical procedure are at risk of thromboembolic events, such as stroke or deep vein thrombosis. Anticoagulation bridging is a strategy used to manage this risk while minimizing the risk of bleeding complications.
What is Anticoagulation Bridging?
Anticoagulation bridging involves temporarily replacing a patient’s long-term anticoagulant medication with a shorter-acting medication, such as heparin or low molecular weight heparin (LMWH), before and after a surgical procedure. The goal of bridging therapy is to reduce the risk of thromboembolic events while minimizing the risk of bleeding complications.
The decision to use bridging therapy depends on several factors, including the patient’s individual risk of thromboembolism and bleeding, the type of surgery being performed, and the specific anticoagulant medication being used. It is important to carefully weigh the risks and benefits of bridging therapy for each patient on an individual basis.
What Does the Research Say?
Several studies have investigated the use of anticoagulation bridging before surgical procedures. A systematic review and meta-analysis published in the Annals of Internal Medicine in 2013 found that bridging therapy was associated with a higher risk of major bleeding complications, but no significant reduction in thromboembolic events. However, the authors noted that the quality of evidence was low and that further research is needed to determine the optimal use of bridging therapy.
A randomized controlled trial published in the New England Journal of Medicine in 2015 compared bridging therapy with a placebo in patients undergoing elective surgery who were at high risk of thromboembolism. The study found no significant difference in the rate of thromboembolic events between the two groups, but a higher rate of major bleeding complications in the bridging therapy group. The authors concluded that routine bridging therapy may not be necessary in patients at high risk of thromboembolism.
Clinical Guidelines:
Clinical guidelines from professional medical associations provide recommendations for the use of anticoagulation bridging before surgical procedures. The American College of Chest Physicians (ACCP) published guidelines in 2012 that recommend against routine bridging therapy in patients at low risk of thromboembolism and suggest individualized assessment of the risks and benefits of bridging therapy in patients at high risk of thromboembolism.
The American College of Cardiology (ACC) and the American Heart Association (AHA) published guidelines in 2014 that recommend against routine bridging therapy in patients with atrial fibrillation who are undergoing elective surgery.
Conclusion:
Anticoagulation bridging before surgical procedures is a strategy used to manage the risk of thromboembolic events in patients who are on long-term anticoagulant therapy. The decision to use bridging therapy should be based on an individualized assessment of the risks and benefits for each patient. While some studies have suggested that routine bridging therapy may not be necessary for all patients, clinical guidelines provide recommendations for the use of bridging therapy in specific patient populations. Further research is needed to determine the optimal use of anticoagulation bridging before surgical procedures.
There are many patients who are receiving long-term treatment with the blood thinner warfarin, whether because of atrial fibrillation or a mechanical heart valve. Such patients frequently require warfarin to be stopped because of an upcoming surgery/procedure. There is uncertainty about whether such patients should receive bridging anticoagulation before and after the surgery/ procedure.
Bridging anticoagulation refers to giving short-acting blood thinner, usually, low-molecular-weight heparin (LMWH) given by subcutaneous injection for 10 to 12 days around the time of the surgery/procedure when warfarin is interrupted and its anticoagulant effect is outside a therapeutic range. Bridging anticoagulation aims to reduce patients’ risk of developing blood clots, such as stroke, but may also increase patients’ risk of developing potentially serious bleeding complications after surgery.
How Is Bridging Anticoagulation Given?
After warfarin is stopped, 5 to 6 days before surgery (to allow sufficient time for its anticoagulant effect to wane), bridging anticoagulation is started 3 days before surgery, with the last dose given 24 hours before surgery. After surgery, bridging is resumed no earlier than 24 hours after surgery; at the same time, warfarin is restarted. Bridging is continued, typically for 4 to 6 days, until the anticoagulant effect of warfarin has resumed and the blood is sufficiently thinned again.
What Low-Molecular-Weight Heparin and What Dose Should Be Used for Bridging?
There is no standardized bridging drug or dose. A therapeutic-dose regimen, for example, enoxaparin (Lovenox) 1 mg/kg twice daily, is often used in North America, although some physicians in other countries use lower doses.
What Happens for Patients Who Are Having a Minor Procedure, Such as a Tooth Extraction or Skin Cancer Removal?
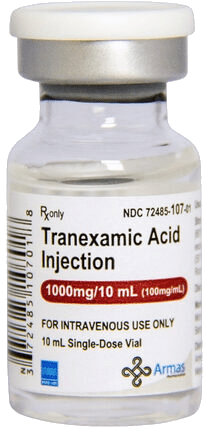
For patients having minor dental work, such as a tooth extraction or root canal, it may not be necessary to stop warfarin. Some dentists allow patients to continue warfarin (especially if there are concerns about stopping it), so long as they take a special mouthwash called tranexamic acid (Amicar) just before and 3 times daily for 1 to 2 days after the dental procedure, to help prevent bleeding. For patients having minor skin procedures or cataract surgery, interruption of warfarin is often not required because there is minimal bleeding.
What Happens for Patients Who Are Having a More Extensive Surgery/Procedure in Which the Bleeding Risk Is High?
In such patients (eg, those having hip or knee replacement or cancer surgery), bridging anticoagulation should be given carefully, especially after surgery. Some physicians may choose to delay the resumption of therapeutic-dose low-molecular-weight heparin bridging for 2 to 3 days after surgery, and others may substitute a lower dose of low-molecular-weight heparin after surgery. There is no single approach, but the intent is to prevent bleeding, typically at the surgical/procedure site. If bleeding occurs, it will further delay the resumption of anticoagulation and will expose patients to an increased risk of blood clots. In other words, if bleeding occurs (perhaps because low-molecular-weight heparin bridging was given too close to surgery), this will have the opposite effect of what bridging was meant to do and can harm patients.
Who Should Receive Bridging Anticoagulation?
This is an unanswered question because there are no completed high-quality clinical studies (referred to as randomized, controlled trials) that tell us who should be bridged. In the meantime, clinical experts have suggested a risk classification scheme to help identify which patients may or may not need bridging (Table), but much work needs to be done.
Summary:
Perioperative hemorrhage is a natural risk of any surgical intervention and deserves a careful evaluation and prompt intervention. However, in order to support ongoing efforts in the prevention of medical errors, the application of evidence-based guidelines for the prophylaxis of VTE in surgical patients must become a standard part of daily practice.
Thromboprophylaxis is an important preventive measure that can significantly reduce the risk of VTE in surgical patients. The choice of thromboprophylaxis depends on various factors such as the type of surgery, patient characteristics, and bleeding risk. Mechanical prophylaxis, pharmacological prophylaxis, and combination prophylaxis are some of the options available. It’s important to discuss the appropriate thromboprophylaxis strategy with each patient prior to surgery.
References:
Lawson, Janice W., Kitchens, Craig S. Surgery and hemostasis: Current Opinion in Hematology https://journals.lww.com/co-hematology/Fulltext/2015/09000/Surgery_and_hemostasis.5.aspx
Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
Kitchens CS. Occult hemophilia. Johns Hopkins Med J 1980; 146:255–259.
Kitchens CS, Lawson JW Surgery and hemostasis. In: Kitchens CS, Kessler CM, Konkle BA, editors. Consultative Hemostasis and Thrombosis, chapter 36, 3rd ed. Philadelphia: Elsevier; 2013. pp. 651–672.
Bates SM, Greer IM, Middeldorp S, et al. Antithrombotic therapy and prevention of thrombosis. ACCP guidelines. Chest 2012; 141 (Suppl 2):691S–736S.
Amin A, Stemkowski S, Lin J, et al. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
Westrich GH. The role of mechanical and other adjuncts. Am J Knee Surg 1999; 12:55–60.
Turpie AGG, Bauer KA, Caprini A, et al. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double-blind comparison. J Thromb Haemost 2007; 5:1854–1861.
Hamilton MG, Yee WH, Hull RD, et al. Venous thromboembolism prophylaxis in patients undergoing cranial neurosurgery: a systematic review and meta-analysis. Neurosurgery 2011; 68:571–581
Bridging Anticoagulation | Circulation. https://www.ahajournals.org/doi/full/10.1161/circulationaha.111.084517
Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S.
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76.
Schulman S, Eriksson H, Schlemmer K, et al. A randomized, controlled trial of dalteparin versus placebo to prevent venous thromboembolism in acutely ill medical patients. Thromb Haemost. 2005;94(5):969-975.
Douketis JD, Johnson JA, Turpie AG. Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen. Arch Intern Med. 2004;164(12):1319-1326.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833.
Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146(4):278-288.
Dentali F, Douketis JD, Lim W, Crowther M, Wells PS. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Intern Med. 2007;167(2):117-124.
Moster, M., Bolliger, D. Perioperative Guidelines on Antiplatelet and Anticoagulant Agents: 2022 Update. Curr Anesthesiol Rep 12, 286–296 (2022). https://doi.org/10.1007/s40140-021-00511-z
Fu JH, McDonald K, Divino CM, Yang J. Optimal timing for resumption of direct oral anticoagulants in patients undergoing elective surgery: a systematic review and meta-analysis. JAMA Surg. 2021;156(5):e210682.
Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330-1393.
Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
Lawson JW, Kitchens CS. Surgery and hemostasis. Curr Opin Hematol. 2015 Sep;22(5):420-7. doi: 10.1097/MOH.0000000000000172. PMID: 26248002.







Cool blog!
amazing…
I want to be a mbbs
Best wishes!
This article has helped me a lot with some good solutions to my queries.
Thank you!