Amyloidosis

Introduction:
Amyloidosis is the name for a group of rare, serious conditions caused by a build-up of amyloid in organs and tissues throughout the body. Amyloid is an abnormal insoluble protein that is produced in the bone marrow and can be deposited in any tissue or organ. The build-up of the amyloid proteins (deposits) can make it difficult for the organs and tissues to work properly. The ICD-10 Code for Amyloidosis is E85. 9.
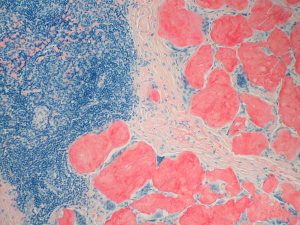
The word “amylon” was first used in 1834 by the German botanist Matthias Schleiden to describe the waxy starch in plants. Rudolph Virchow then coined the word “amyloid” in 1854 to describe tissue deposits that stained like cellulose when exposed to iodine. Pathologists now know that amyloid, using the Congo red stain introduced in the 1920s, looks pink with normal lighting and demonstrates apple-green birefringence under polarized light. The various types of amyloid are indistinguishable using light microscopy. It is therefore essential for the pathologist to perform additional studies to definitively identify the type of protein involved since the prognosis and treatment of amyloidosis can vary widely depending on the protein responsible.
Amyloidosis can affect different organs in different people, and there are different types of amyloid. Amyloidosis frequently affects the heart, kidneys, liver, spleen, nervous system and digestive tract. Without treatment, severe amyloidosis can lead to life-threatening organ failure. There’s no cure for amyloidosis, but treatments can help to manage the symptoms and limit the production of amyloid protein.
Types of Amyloidosis:
AL Amyloidosis (immunoglobulin light chain amyloidosis):
The most common type of amyloidosis and used to be called primary amyloidosis results when light chains are produced in excess by clonal or frankly malignant plasma cells. It occurs in 10-15% of patients with full-blown multiple myeloma but can also be seen when the affected patient has less than 10% bone marrow plasma cells, the quantity typically required to make a diagnosis of myeloma. Light-chain amyloidosis may also arise in association with non-Hodgkin’s lymphoma and Waldenström’s macroglobulinemia. It is a relatively rare disease with an incidence of 5.1-12.8 per million person-years and only 1,275-3,200 new cases being diagnosed in the United States annually. The male-to-female ratio is 3:2. Although healthy individuals have a preponderance of kappa free light chains (K/λ) = 2:1), the reverse is true in most patients with primary amyloidosis, as excess lambda light chains have a greater propensity to be amyloidogenic.
AA amyloidosis:
Previously known as secondary amyloidosis, this condition is the result of another chronic infectious or inflammatory diseases such as rheumatoid arthritis, Crohn’s disease, or ulcerative colitis. It mostly affects the kidneys, but it can also involve the digestive tract, liver, and heart. AA means the amyloid type A protein causes this type.
Dialysis-related amyloidosis (DRA):
This is more common in older adults and people who have been on dialysis for more than 5 years. This form of amyloidosis is caused by deposits of beta-2 microglobulin that build up in the blood. Deposits can build up in many different tissues, but it most commonly affects bones, joints, and tendons.
Familial, or hereditary, amyloidosis:
This is a rare form passed down through families. It often affects the liver, nerves, heart, and kidneys. Many genetic defects are linked to a higher chance of amyloid disease. For example, an abnormal protein like transthyretin (TTR) can be the cause.
Age-related (senile) systemic amyloidosis:
This is caused by deposits of normal TTR in the heart and other tissues. It happens most commonly in older men.
Organ-specific amyloidosis:
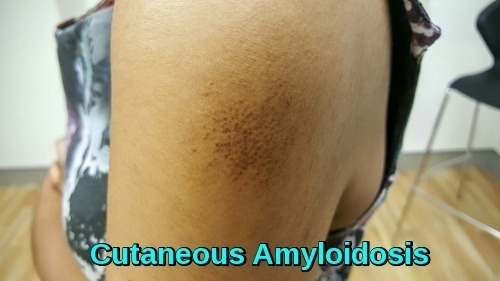
This causes deposits of amyloid protein in single organs, including the skin (cutaneous amyloidosis). Though some types of amyloid deposits have been linked to Alzheimer’s disease, the brain is rarely affected by amyloidosis that happens throughout the body.
Amyloidosis symptoms and signs:
Amyloidosis presents in a variety of ways and can make diagnosis difficult. It is helpful to separate the presentation into two patterns: localized and systemic. Localized amyloidoses are confined to one site and are generally non-lethal. Skin is a common site of deposition and can manifest as asymptomatic plaques, fissures or nodules.
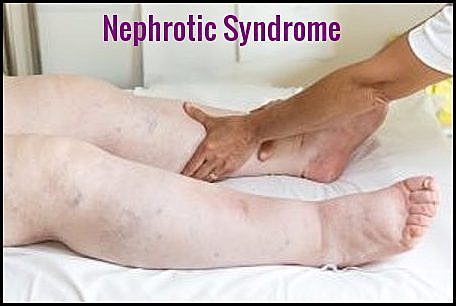
Systemic amyloidosis implies the involvement of visceral organ(s) or multiple tissues. Patients may present with symptoms limited to one organ or with multiorgan failure. The kidney is the most common organ involved in AL, AA and most forms of hereditary amyloidosis except ATTR. Proteinuria is present in 73% of the AL amyloidosis patients with 30% exhibiting nephrotic syndrome. Renal insufficiency is noted in nearly half of the patients which is usually associated with proteinuria. Renal involvement is nearly
universal (97%) in AA amyloidosis.
The heart is the next most common organ involved in AL amyloidosis with abnormal echocardiographic findings noted in 65% of patients. Presentation varies from asymptomatic to a subtle decreased in exercise capacity to fatigue, dyspnea and lower extremity edema to angina, syncope, ascites, and anasarca which are associated with more advanced disease. Overt heart failure can be seen in 24%. Low voltage on electrocardiogram (ECG) and concentric thickened ventricles on echocardiogram are classic signs of cardiac involvement by amyloidosis. Cardiac involvement, on the other hand, is rare in AA amyloidosis occurring in only 1% of patients.
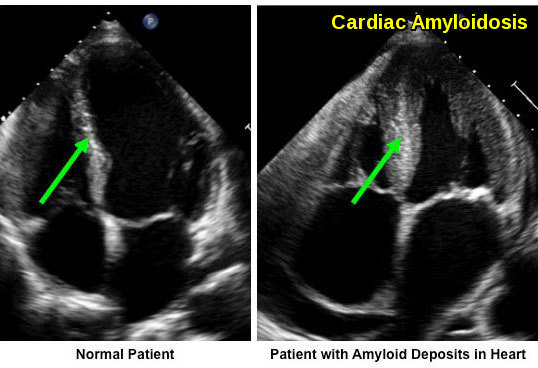
Amyloid fibrils make the heart’s walls appear thicker when they accumulate in the heart. Notice how much thicker the wall is in the patient with amyloidosis.
The nervous system is a prominent feature in some forms of amyloidosis and can involve the central, peripheral nerves or both. Peripheral nerve involvement can present with bilateral distal progressive paraesthesia or carpel tunnel syndrome. Autonomic nervous
system involvement is characterized by syncope, erectile dysfunction, gastroparesis, and diarrhea.
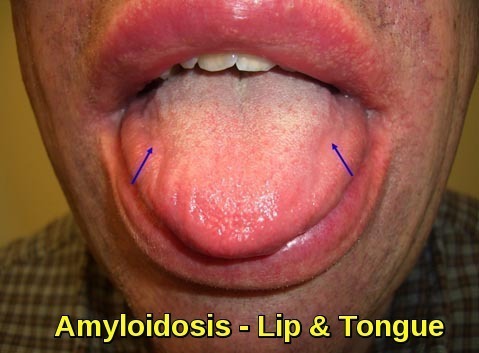
Gastrointestinal presentation is not common and usually present with macroglossia, nausea, vomiting, and pseudoobstruction which accounts for < 10% of AL amyloidosis cases. Hepatomegaly is present in 24% of AL amyloidosis patients but only 9% of AA
amyloidosis. Bruising is common and often occurs after minor trauma or procedure especially around the eyes.
Symptoms common to most types of amyloid include fatigue and weight loss which are reported by over half of the patients. Weight loss, however, is unreliable in patients with nephrotic syndrome since many will gain weight as their edema worsens.
In summary, symptoms in patients with amyloidosis result from abnormal functioning of the particular organs involved. There might be no symptoms until the disease is relatively advanced. The heart, kidneys, liver, bowels, skin, nerves, joints, and lungs can be affected. As a result, symptoms and signs are vague and can include fatigue, shortness of breath, weight loss, lack of appetite, numbness, tingling, carpal tunnel syndrome, weakness, hearing loss, enlarged tongue, bruising, and swelling of hands and feet. Amyloidosis in these organs leads to cardiomyopathy, heart failure, peripheral neuropathy, arthritis, malabsorption, diarrhea, and liver damage and failure. Amyloidosis affecting the kidney leads to “nephrotic syndrome” which is characterized by severe loss of protein in the urine and swelling of the extremities.
Diagnosis:
The diagnosis of amyloidosis is often delayed because the symptoms are so varied. Patients often get treated for heart or kidney failure for months before the underlying root of the problem is identified, typically by a biopsy.
Blood and urine tests may reveal an abnormal immunoglobulin protein in the body in those patients with AL Amyloidosis, but the only way to diagnose amyloidosis for certain is to take a sample of tissue for analysis under a microscope. The tissue is often taken from the fat around the abdomen which can be done on an outpatient basis in patients suspected as having the condition. This fat aspiration test will show a confirmatory positive result in 80% of patients with amyloidosis. Alternatively, a biopsy of the organ that is not functioning properly such as the heart, kidney or nerve can more reliably identify the condition but can be more cumbersome to obtain. This method of identification is becoming more common in our experience and often leads to the diagnosis in patients not suspected of the condition beforehand. A biopsy of the bone marrow is another useful method of detection and is critical to perform in patients suspected as having AL amyloidosis since it can identify and quantify the bone marrow lymphocytes or plasma cells which typically live there and are causing the problem. All of the above samples are stained with a dye called Congo Red that reacts with amyloid which can then be identified under a microscope.
It is critical to characterize the nature of the amyloid protein that is present since each of the various types of amyloidosis (such as AL, ATTR, or AA) is treated quite differently. Mass spectroscopy is a special technique that can be used on tissue samples to reliably determine the type of protein present. Blood samples can be used to sequence the various genes such as transthyretin or fibrinogen to determine if a predisposing gene variant exists. For patients with AL Amyloidosis, the free light chain assay is extremely useful to monitor the response to treatment. Patients suspected of heart involvement can get a cardiac MRI which can typically demonstrate findings suggestive of amyloidosis in those patients afflicted.
Treatment of Amyloidosis:
Treatment of amyloidosis is given to improve symptoms and extend life. Treatment can limit further production of amyloid proteins and, in some instances, promote the breakdown of amyloid proteins in affected organs. The type of treatment required varies depending on the type of amyloidosis and the patient’s symptoms.
With secondary amyloid, the main goal of therapy is to treat the underlying condition — for example, taking an anti-inflammatory medication for rheumatoid arthritis or antibiotics for an infection.
In hereditary amyloid, liver transplantation has been the most effective therapy. The new liver does not produce the abnormal amyloid proteins and consequently, the disease improves. Investigational drugs are also being evaluated to try and prevent this type of amyloid protein from depositing in organs.
For primary amyloid, treatments include the same agents used to treat multiple myeloma, such as chemotherapy, corticosteroids, immunomodulatory drugs (lenalidomide or thalidomide) and/or bortezomib (Velcade). These treatments slow organ deterioration and some have been shown to prolong life, but none provide a cure.
Because primary amyloid is such a difficult disease to treat and survival is limited, researchers have begun to investigate the use of high-dose chemotherapy with autologous stem cell transplantation as a means of prolonging survival. The initial results with autologous stem cell transplantation are encouraging.
Autologous Stem Cell Transplantation
Patients with primary amyloid undergo an extensive workup to evaluate organ function and the effects that amyloidosis has had on the body. Those with adequate heart, liver and lung function are encouraged to proceed to autologous stem cell transplantation.
High-dose melphalan chemotherapy is administered over one day. Then the patient’s own stem cells (bone marrow) are re-administered two to three days later. An additional three to four weeks are spent in the hospital awaiting recovery and growth of the bone marrow.
The hope is that this therapy will delay the progression of the disease, and in some cases, improve symptoms through the removal of the abnormal proteins from the organs. However, this therapy is not a cure, and amyloidosis will return in everyone. That said, we have had patients who have been successfully treated with stem cell transplantation and when their disease progressed, have been able to receive another stem cell transplant.
Several new investigational agents are being evaluated in the treatment of multiple myeloma, another plasma cell disorder. The hope is that some of these agents also may be effective in treating amyloidosis. For patients who are not candidates for stem cell transplantation, these agents may prove to be the best available treatment.
Patients with hereditary amyloid should be referred to a Liver Transplant Clinic for evaluation.
References:
Baker KR, Rice L. The amyloidoses: clinical features, diagnosis and treatment. Methodist Debakey Cardiovasc J. 2012;8(3):3–7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3487569/
Nelson Leung, Samih H. Nasr and Sanjeev Sethi. How I diagnose amyloidosis. http://www.bloodjournal.org/content/bloodjournal/early/2012/09/04/blood-2012-03-413682.full.pdf?sso-checked=true
Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. New England Journal of Medicine. 2003;349(6):583-596.
Amyloidosis – Stanford Health Care. https://stanfordhealthcare.org/medical-conditions/blood-heart-circulation/amyloidosis.html
Guidelines on the diagnosis and investigation of AL amyloidosis – Gillmore – 2015 – British Journal of Haematology – Wiley Online Library https://onlinelibrary.wiley.com/doi/full/10.1111/bjh.13156
Amyloidosis – Symptoms and causes – https://www.mayoclinic.org/diseases-conditions/amyloidosis/symptoms-causes/syc-20353178
Kyle RA. Amyloidosis: a convoluted story. Br J Haematol. 2001 Sep;114(3):529–38.
Lachmann HJ, Booth DR, Booth SE, Bybee A, Gilbertson JA, Gillmore JD, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002 Jun 6;346(23):1786–91.
Comenzo RL. How I treat amyloidosis. Blood. 2009 Oct 8;114(15):3147–57.
Falk RH, Comenzo RL, Skinner M. The systemic amyloidosis. N Engl J Med. 1997 Sep 25;337(13):898–909.
Dubrey SW, Cha K, Andersen J, Chamarthi B, Reisinger J, Skinner M, et al. The clinical feature of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998 Feb;91(2):141–57.
William C. Shiel Jr. Amyloidosis https://www.medicinenet.com/amyloidosis/article.htm
Amyloidosis: Symptoms, Treatments, Prognosis, Causes. https://wb.md/2ziYn7i
from @WebMD
Diagnosis of Amyloidosis. https://www.cedars-sinai.org/programs/cancer/we-treat/multiple-myeloma-and-amyloidosis/conditions/diagnosis.html#
Treatment of Amyloidosis – University of California San Francisco. https://www.ucsfhealth.org/conditions/amyloidosis/
Keywords:
amyloidosis symptoms, amyloidosis diagnosis, amyloidosis causes, amyloidosis meaning, amyloidosis heart, amyloidosis icd 10, amyloidosis treatment, amyloidosis types, amyloidosis symptoms and signs, amyloidosis pictures, amyloidosis symptoms heart, amyloidosis symptoms skin, amyloidosis symptoms liver, amyloidosis symptoms nerves, amyloidosis symptoms bowel, amyloidosis symptoms kidneys, renal amyloidosis, amyloidosis diagnosis criteria, amyloidosis diagnosis test, amyloidosis diagnosis labs, amyloidosis diagnosis life expectancy, amyloidosis prognosis, amyloidosis diagnosis and treatment, amyloidosis causes death, cardiac amyloidosis causes, amyloidosis heart disease, amyloidosis heart transplant, amyloidosis heart scan.