Hemochromatosis
Hereditary Hemochromatosis (HH) is a genetic disorder that causes the body to absorb too much iron (Fe) from the diet. The excess iron is stored in the body’s tissues and organs, particularly the skin, heart, liver, pancreas, and joints.
Because humans cannot increase iron excretion, excess iron can overload and eventually damage tissues and organs. For this reason, hereditary hemochromatosis is also called an iron overload disorder.
Early symptoms of HH are nonspecific and may include fatigue, joint pain, abdominal pain, and loss of sex drive. Later signs and symptoms can include arthritis, liver disease, diabetes, heart abnormalities, and skin discoloration.
Although penetrance is more commonly an issue with autosomal dominant disorders, it appears to be an issue in hereditary hemochromatosis. Thirty to fifty percent of those with homozygous genotypes do not have clinical evidence of hemochromatosis. Many factors, including alcohol consumption, dietary iron intake, blood loss associated with menstruation and pregnancy and blood donation, influence the expression.
Hereditary hemochromatosis is classified by type depending on the age of onset and other factors such as genetic cause and mode of inheritance.
There are 4 types of HH, types 1 through 4.
Type 1, the most common form of the disorder, and type 4 (also called ferroportin disease) begin in adulthood. Men with type 1 or type 4 hemochromatosis typically develop symptoms between the ages of 40 and 60, and women usually develop symptoms after menopause.
Type 2 hemochromatosis is a juvenile-onset disorder. Iron accumulation begins early in life, and symptoms may appear in childhood. By age 20, decreased or absent secretion of sex hormones is evident. Females usually begin menstruation in a normal manner, but menses stop after a few years. Males may experience delayed puberty or symptoms related to a shortage of sex hormones. If the disorder is untreated, heart disease becomes evident by age 30.
The onset of type 3 hemochromatosis is usually intermediate between types 1 and 2. Symptoms of type 3 hemochromatosis generally begin before age 30.
Genetics:
Type 1 Hereditary Hemochromatosis results from a genetic mutation to the HFE Gene on Chromosome 6 and may involve the C282Y, H63D, or S65C alleles. This type is one of the most common genetic disorders in the United States, affecting about 1 million people. It most often affects people of Northern European descent. The other types of hemochromatosis are rare and have been studied in only a few families worldwide.
Mutations in several genes, including HAMP, HFE, HFE2, SLC40A1, and TFR2, can cause hereditary hemochromatosis. Type 1 hemochromatosis results from mutations in the HFE gene, and type 2 hemochromatosis results from mutations in either the HFE2 or HAMP gene. Mutations in the TFR2gene cause type 3 hemochromatosis, and mutations in the SLC40A1 gene cause type 4 hemochromatosis.
The proteins produced from these genes play essential roles in regulating iron absorption, transport, and storage. Mutations in any of these genes impair the control of iron absorption during digestion and alter iron distribution to other body parts. As a result, iron accumulates in tissues and organs, which can disrupt their normal functions.
Types 1, 2, and 3 hemochromatosis are inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. Most often, the parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene but do not show signs and symptoms of the condition.
Type 4 hemochromatosis is distinguished by its autosomal dominant inheritance pattern. With this type of inheritance, one copy of the altered gene in each cell is sufficient to cause the disorder. In most cases, an affected person has one parent with the condition.
Who Inherits Hereditary Hemochromatosis?
Type 1 hereditary hemochromatosis most commonly results from a homozygous gene mutation or a compound heterozygous gene mutation. A homozygous gene refers to two mutations of the same allele, whereas a heterozygous gene indicates one allele mutation.
Since several alleles affect iron absorption (C282Y, H63D, or S65C), another possibility is having two different heterozygous mutations simultaneously. When this occurs, an individual is known as a compound heterozygote.
A summary of the most common HFE Gene Mutation patterns:
Homozygote for 2 “Missense” Copies of the C282Y Allele.
Compound Heterozygote for 1 “Missense” Copy of C282Y and 1 of H63D or S65C.
Indeed, when a person’s genetic profile possesses two mutations of the HFE gene (as described above), their health is at high risk of iron overload.
By the numbers, 1 in 200 people of Northern European descent have two genetic mutations of the HFE gene. This includes homozygous individuals for two copies of the C282Y gene and compound heterozygote individuals (C282Y + H63D).
Additionally, the numbers tell us that 1 in 9 individuals of Northern European descent are carriers of one HFE gene. This means that having a single hemochromatosis gene is not uncommon!
Pathophysiology:
The body typically stores iron from the diet in the bone marrow, with small amounts stored in the liver (to form new red blood cells).
Normal body iron content is about 2.5 g in women and 3.5 g in men. Because symptoms may be delayed until iron accumulation is excessive (e.g. > 10 to 20 g), hemochromatosis may not be recognized until later in life, even though it is an inherited abnormality. Clinical manifestations are uncommon in women before menopause because iron loss due to menses (and sometimes pregnancy and childbirth) tends to offset iron accumulation.
The mechanism for iron overload in both HFE and non-HFE hemochromatosis is increased iron absorption from the GI tract, leading to chronic iron deposition in the tissues. Hepcidin, a liver-derived peptide, is the critical control mechanism for iron absorption. Hepcidin is commonly upregulated when iron stores are elevated and, through its inhibitory effect on ferroportin (which participates in iron absorption), it prevents excessive iron absorption and storage in ordinary people. Hemochromatosis types 1 through 4 share the same pathogenic basis (lack of hepcidin synthesis or activity) and key clinical features.
 In general, tissue injury appears to result from reactive free hydroxyl radicals generated when iron deposition in tissues catalyzes their formation. Other mechanisms may affect particular organs (e.g. skin hyperpigmentation can result from increased melanin as well as iron accumulation). In the liver, iron-associated lipid peroxidation induces hepatocyte apoptosis, which stimulates Kupffer cell activation and release of pro-inflammatory cytokines. These cytokines activate hepatic stellate cells to produce collagen, resulting in pathologic accumulation of liver fibrosis.
In general, tissue injury appears to result from reactive free hydroxyl radicals generated when iron deposition in tissues catalyzes their formation. Other mechanisms may affect particular organs (e.g. skin hyperpigmentation can result from increased melanin as well as iron accumulation). In the liver, iron-associated lipid peroxidation induces hepatocyte apoptosis, which stimulates Kupffer cell activation and release of pro-inflammatory cytokines. These cytokines activate hepatic stellate cells to produce collagen, resulting in pathologic accumulation of liver fibrosis.
The clinical consequences of iron overload are the same regardless of the etiology and pathophysiology of the overload.
Early symptoms are often nonspecific, so it is important to consider hemochromatosis as a diagnostic consideration for nondescript symptoms like chronic fatigue, features of diabetes mellitus, arthralgia and loss of libido. The most common presenting symptoms for hereditary hemochromatosis are weakness, abdominal pain, or joint pains. Many cases are asymptomatic. Northern European ancestry is important, and the frequency for hereditary hemochromatosis is highest in Northern European populations or descendants. Italians, Greeks and Ashkenazi Jews are at lower risk as these populations have a lower allele frequency.
Historically, experts believed that symptoms did not develop until significant organ damage had occurred. However, organ damage is slow and subtle, and fatigue and nonspecific systemic symptoms and signs often occur early. For example, liver dysfunction can manifest insidiously with fatigue, right upper quadrant abdominal pain, and hepatomegaly. Laboratory abnormalities of iron overload and hepatitis usually precede symptoms.
 In type 1 hereditary (HFE) hemochromatosis, symptoms relate to the organs with the largest iron deposits. In men, the initial symptoms may be hypogonadism and erectile dysfunction caused by gonadal iron deposition. Glucose intolerance or diabetes mellitus is another common initial presentation. Some patients present with hypothyroidism.
In type 1 hereditary (HFE) hemochromatosis, symptoms relate to the organs with the largest iron deposits. In men, the initial symptoms may be hypogonadism and erectile dysfunction caused by gonadal iron deposition. Glucose intolerance or diabetes mellitus is another common initial presentation. Some patients present with hypothyroidism.
Liver disease is the most common complication and may progress to cirrhosis; 20 to 30% of patients with cirrhosis develop hepatocellular carcinoma. Liver disease is the most common cause of death.


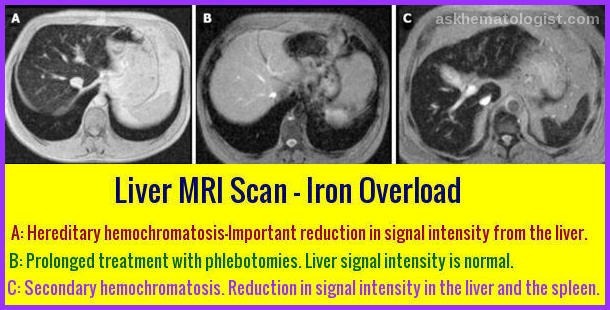 Cardiomyopathy with heart failure ± arrhythmias is the 2nd most common fatal complication. As well as building up in other organs of the body, iron levels can build up in the heart. This happens slowly, and the heart may continue to work well until the overload becomes quite advanced. This can cause restrictive or dilated cardiomyopathy.
Cardiomyopathy with heart failure ± arrhythmias is the 2nd most common fatal complication. As well as building up in other organs of the body, iron levels can build up in the heart. This happens slowly, and the heart may continue to work well until the overload becomes quite advanced. This can cause restrictive or dilated cardiomyopathy.
 Hyperpigmentation and porphyria cutanea tarda are common, as is symptomatic arthropathy (particularly affecting the fingers).
Hyperpigmentation and porphyria cutanea tarda are common, as is symptomatic arthropathy (particularly affecting the fingers).
Joint abnormalities are common and often lead to morbidity and loss of quality of life. Joint disease often is an early manifestation of hemochromatosis and may be the symptom complex that leads to hemochromatosis diagnosis; however, a high index of suspicion must be maintained because the joint findings mimic those seen in other common rheumatologic conditions. An arthropathy associated with hemochromatosis was first described by Schumacher in 1964. The rate of occurrence of arthritis in persons with hemochromatosis has been variously estimated depending on the design of the study. Rates of about 50% have been reported when rigorous criteria are used to define arthritis, and rates have varied in other survey-based studies.
 Hemochromatosis is sometimes referred to as bronze diabetes because it can lead to darkening/tanning of the skin and hyperglycemia with thirst and increased urination. Hyperpigmentation may be generalized but is often more apparent in sun-exposed areas.
Hemochromatosis is sometimes referred to as bronze diabetes because it can lead to darkening/tanning of the skin and hyperglycemia with thirst and increased urination. Hyperpigmentation may be generalized but is often more apparent in sun-exposed areas.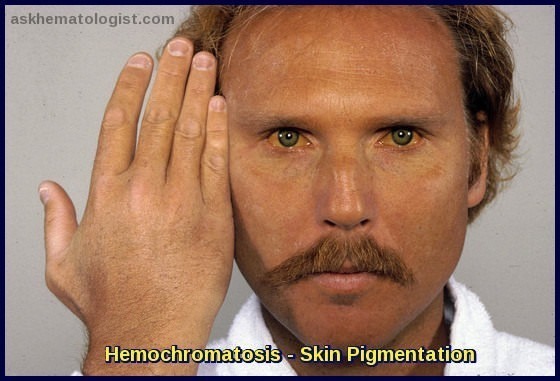 In type 2 disease, symptoms and signs include progressive hepatomegaly and hypogonadotropic hypogonadism.
In type 2 disease, symptoms and signs include progressive hepatomegaly and hypogonadotropic hypogonadism.In type 3 disease, symptoms and signs are similar to type 1 hereditary (HFE) hemochromatosis.
Type 4 disease manifests in the first decade of life as increased serum ferritin levels with low or normal transferrin saturation; progressive saturation of transferrin occurs when patients are in their 20s and 30s. Clinical manifestations are milder than in type 1 disease, with modest liver disease and mild anemia.
Transferrin and ceruloplasmin deficiency:
In transferrin deficiency (hypotransferrinemia or atransferrinemia), absorbed iron that enters the portal system not bound to transferrin is deposited in the liver. Subsequent iron transfer to sites of RBC production is reduced because of transferrin deficiency.
In ceruloplasmin deficiency (aceruloplasminemia), lack of ferroxidase causes defective conversion of Fe2+ to Fe3+; such conversion is necessary for binding to transferrin. Defective transferrin binding impairs the movement of iron from intracellular stores to plasma transport, resulting in the accumulation of iron in tissues.
Diagnosis:
Early diagnosis of hemochromatosis is essential to prevent irreversible organ damage. The diagnostic workup includes a thorough medical history, physical examination, and laboratory tests. Key laboratory findings include elevated serum ferritin, increased transferrin saturation, and elevated liver enzyme levels. Genetic testing for HFE gene mutations can help confirm the diagnosis and identify family members at risk.
Since hemochromatosis potentially involves more than one organ system, it is important to refer a suspected case to a family physician or general internist who can then coordinate examinations, testing, and results from various specialities, as well as genetic testing.
Liver, pancreatic, cardiac, and joint disease should be confirmed by physical examination, radiography, and standard functional tests for these organs. It is probable that a patient with hemochromatosis may need care from rheumatology, endocrinology, cardiology, gynecology, pathology and hematology in addition to dermatology due to the potential for iron build-up in parenchymal tissues.
Iron stores can be assessed by measurement of serum iron, the percent saturation of transferrin, and measurement of serum ferritin concentration. The percent transferrin saturation and serum ferritin level provide a simple and reliable screening test for hemochromatosis, including the pre-cirrhotic phase of the disease.If either test is abnormal two or more times, the patient should be referred for genetic testing.
Fasting Transferrin Saturation: A fasting transferrin saturation persistently greater than 45-50% (reference is 15-50% for men and 12-45% for women) is the earliest marker of iron overload. Patients with elevated transferrin saturation on two or more occasions should have the HFE gene test.
Serum Ferritin: The reference range is 12-150ng/mL for women and 12-300ng/mL for men. Ferritin reflects increased iron stores, but it may also increase with alcohol consumption, liver disease, and acute illness. If ferritin is elevated, it is recommended to take another sample, ensuring the patient has been fasting, to assess levels a second time. If they are still elevated on repeat, then referral for HFE gene testing is recommended, particularly if transferrin saturation is also high or borderline.
Genetic Testing: Polymerase chain reaction-based methods can be used to diagnose a hereditary form of hemochromatosis. DNA may be obtained from blood or from buccal cells. Hereditary hemochromatosis is diagnosed in patients with iron overload if the HFE gene test shows either C282Y homozygosity or C282Y/H63D compound heterozygosity. A negative genetic test does not disprove the diagnosis.
HFE gene testing should be performed in all patients presenting with porphyria cutanea tarda.
All adult first-degree relatives of patients with hereditary hemochromatosis should be tested for the C282Y and H63D mutations. Homozygosity at the C282Y position of the HFE gene accounts for the majority of hemochromatosis cases. Genetic testing should be performed with informed consent and appropriate counselling.
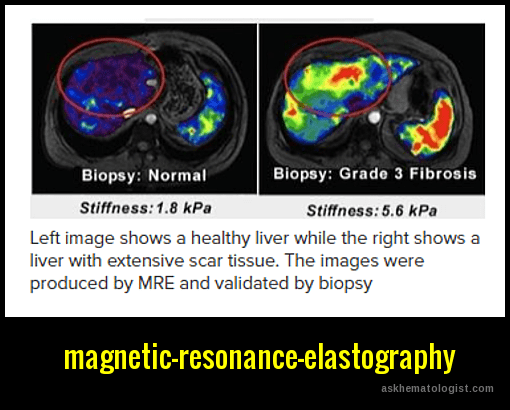
Biopsy and Magnetic Resonance Elastography: Today, magnetic resonance elastography is used to assess fibrosis instead of liver biopsy. However, if this imaging method is unavailable, liver biopsy will show surplus iron visible on histologic examination of hepatic tissue, particularly after staining with potassium ferrocyanide.
Skin biopsies also show characteristic changes. Increased melanin and hemosiderin is most often found in sweat glands, but blood vessels and dermal tissue may also contain deposits. Establishing or excluding the presence of hepatic cirrhosis is critical in determining prognosis and the risk of developing hepatocellular carcinoma. Results of liver biopsy do not alter treatment strategy.
ICD-10 code:
The ICD-10 code for hemochromatosis is E83.1. This code is used to classify and code for the disorder in the International Classification of Diseases, 10th Edition (ICD-10).
Treatment:
Phlebotomy:
Treatment is indicated for patients with clinical manifestations, elevated serum ferritin levels (particularly levels > 1000 ng/mL), or elevated transferrin saturation. Asymptomatic patients need only periodic (e.g. yearly) clinical evaluation and measurement of serum iron, ferritin, transferrin saturation, and liver enzymes.
Phlebotomy (venesection) is the simplest and most effective method to remove excess iron. It delays the progression of fibrosis to cirrhosis, sometimes even reversing cirrhotic changes, and prolongs survival, but it does not prevent hepatocellular carcinoma.
About 500 mL of blood (about 250 mg of iron) is removed weekly or biweekly (every other week) until serum ferritin levels reach 20 to 50 ng/mL. Weekly or biweekly phlebotomy may be needed for many months (e.g. if 250 mg of iron are removed per week, 40 weeks will be required to remove 10 g of iron). When iron levels are normal, phlebotomies can be intermittent to maintain ferritin between 50 and 100 ng/mL.
Patients should follow a balanced diet; it is not necessary to restrict consumption of iron-containing foods (e.g. red meat, liver). Alcohol should be consumed only in moderation because it can increase iron absorption and, in high amounts, increases the risk of cirrhosis. Vitamin C supplements should be avoided.
In patients with type 4 disease, tolerance to vigorous phlebotomy is poor; serial monitoring of Hb level and transferrin saturation is required.
Treatment of transferrin deficiency and ceruloplasmin deficiency is experimental; e.g. iron chelators may be better tolerated than phlebotomy because patients typically have anemia.
Chelating Agents:
When anemia or hypoproteinemia is severe, chelating agents, like Deferoxamine (Desferal), may be useful. Deferasirox (Exjade) is a newer oral chelating agent that is effective in thalassemia and secondary iron overload. Its role in primary iron overload has yet to be established. Deferoxamine may also be effective, but the medication can be inconvenient, due to the method of administration, and expensive.
Addressing Organ Damage:
Referral to respective specialists is recommended. Loss of libido and change in secondary sex characteristics are usually managed with hormone replacement or gonadotropin therapy. The degree of cardiac involvement may be assessed by radiography, electrocardiography and other cardiac tests before initiating the venesection treatments. Alcohol consumption should be avoided completely since it increases the risk of cirrhosis.
Conclusion:
Hemochromatosis poses a significant health burden due to the potential for irreversible organ damage if not diagnosed and managed early. A high index of clinical suspicion, supported by appropriate laboratory and genetic testing, is essential for early detection. Phlebotomy remains the cornerstone of treatment, while newer therapeutic options are under investigation. Genetic counselling and family screening are critical to preventing complications in at-risk individuals.
References:
Haemochromatosis Logo Image: https://www.haemochromatosis.org.uk/
Pietrangelo A: Hereditary hemochromatosis: Pathogenesis, diagnosis, and treatment. Gastroenterology 139:393–408, 2010.
James Peter Adam Hamilton, MD. Hereditary Hemochromatosis – Hematology and Oncology – Merck Manuals Professional Edition http://www.merckmanuals.com/professional/hematology-and-oncology/iron-overload/hereditary-hemochromatosis. Last accessed January 2017.
Genetics Home Reference: hereditary hemochromatosis https://ghr.nlm.nih.gov/condition/hereditary-hemochromatosis#
Type 1 Hereditary Hemochromatosis & HFE Gene | Hemochromatosis Help http://hemochromatosishelp.com/type-1-hereditary-hemochromatosis
Castiella A, Zapata E, Alústiza JM. Non-invasive methods for liver fibrosis prediction in hemochromatosis: One step beyond. World J Hepatol 2010; 2(7): 251-255
Mark R. Pittelkow, Sara Flores. Hemochromatosis http://www.clinicaladvisor.com/dermatology/hemochromatosis/article/588242/
Mayo Clinic Pioneers Sound Waves to Diagnose Disease https://singularityhub.com/2010/10/06/mayo-clinic-pioneers-sound-waves-to-diagnose-disease/
Schumacher HR Jr. Hemochromatosis and arthritis. Arthritis Rheum. 1964,7:41-50.
Adams P, Brissot P, Powell LW. EASL International Consensus Conference on Haemochromatosis. J Hepatol. 2000;33(3):485-504.
Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343.
Pietrangelo A. Hereditary hemochromatosis – a new look at an old disease. N Engl J Med. 2004;350(23):2383-2397.
Keywords:
Hemochromatosis and alcohol, hemochromatosis and diabetes, hemochromatosis and pregnancy, hemochromatosis arthritis, hemochromatosis and liver, hemochromatosis age of onset, hemochromatosis and vitamin c, hemochromatosis blood test, hemochromatosis blood donation, hemochromatosis bronze skin, hemochromatosis carrier, hemochromatosis ICD 10, hemochromatosis cardiomyopathy, hemochromatosis cure, hemochromatosis cirrhosis, hemochromatosis complications, hemochromatosis cancer, hemochromatosis diagnosis, hemochromatosis diet, hemochromatosis doctor, haemochromatosis death rate, haemochromatosis DNA test, haemochromatosis erectile dysfunction, haemochromatosis elevated liver enzymes, haemochromatosis echo, haemochromatosis foods to avoid, haemochromatosis ferritin level goal, haemochromatosis fatigue, haemochromatosis gene, haemochromatosis hereditary, haemochromatosis heterozygous, haemochromatosis heart, haemochromatosis hfe gene, haemochromatosis h63d, hemochromatosis iron levels, haemochromatosis joint pain, hemochromatosis joints, haemochromatosis knuckles, haemochromatosis muscle pain, haemochromatosis normal ferritin, haemochromatosis one gene, haemochromatosis organ damage, haemochromatosis phlebotomy, hemochromatosis venesection, haemochromatosis pictures, hemochromatosis recessive, haemochromatosis risk factors, haemochromatosis symptoms, haemochromatosis treatment, haemochromatosis test, haemochromatosis types, haemochromatosis transferrin saturation, haemochromatosis testosterone, haemochromatosis untreated, hemochromatosis vitamins to avoid, hemochromatosis vitamin d deficiency, hemochromatosis and zinc.









I have been on therapeutic phlebotomies for 3.5 months.
My original ferritin level was 1700, but for the last 4 weeks I have plateaued in the 700s and seem to be climbing again. My lowest was 702, but I am now back at 793.
I am very careful with my diet, have changed my MVI to a non iron formula
I am extremely exhausted after the phlebotomies and experience severe foot and leg cramps the next day despite drinking. I have called my hematologist office 4 times within the last week and have had no return call.
I am concerned about the plateauing and the cramping. I am a nurse and this impacts my ability to care for my patients
I also had an episode where every needle placed in my vein immediately clotted and now one vein is not usable for venipuncture, Labs were done, but nothing was outstanding except the D Dymer which was slightly elevated (400). A lower extremity U/S was performed and read as negative. My only remaining vein is becoming scarred and hard to access. There was talk about placing a port, but nothing finalized yet.
Do you have any further ideas?
I am wondering do I need a second opinion or is this normal progression?
My hemochromatosis was found incidentally this past summer during yearly lab work required s/p gastric sleeve surgery. I was then found to have a gall stone in the common bile duct which prompted my gallbladder to be removed and a liver bx done in July. There is no known family history.
I am a 54 year old active 132 pound female-non menstruating.
My WBC range from 2.8-3.2-not getting clear answers as to why they are low.
I have already experience the joint destruction have 2 joints replaced in the last 11 years (hip and knuckle)
I guess my question is-do I need a second opinion or is this the normal progression of the disorder?
Thank you!
Hi Kimberly,
Thank you for your message.
Ferritin is an acute phase reactant which could rise in response to inflammation, infection or malignancy. Alcohol can also affect your serum ferritin level.
If your ferritin is persistently raised in spite of venesections, you may check your fasting serum iron and iron saturation.
You haven’t mentioned if your hemochromatosis is genetic or secondary?
Can you send me a copy of your HFE genetic test to have a look?
Regards,
Author
Date of Last Result Test Result Reference Range Flag
Mar 14, 2019 Ferritin 243.8 ng/ml < only test run
These below were done in Dec of 2018
Dec 28, 2018 Iron Level 174 ug/dl 50-170 High
total iron binding 223 ug/dl 250-450 ug/dl Low
Transferrin % Saturation 78.0% 15-50% High
Transferrin 169 mg/dl 212-360 mg/dl Low
I see a Hematologist every 6 months due to breast cancer 2 years back. I don't like these numbers
and I'm concerned. He says I"m good. I have genetic Hemochromatosis I have Osteoporosis and now experiencing some discomfort in my right side where my liver is.
Hello,
Thank you for your message.
If you have confirmed genetic Hemochromatosis and feel discomfort at your liver site I would recommend to check your liver functions and to have an MRI scan on your liver if available. You should also be on regular venesections if your transferrin saturation (TS) is 78%.
TS is a more reliable index in Hemochromatosis compared to ferritin which could rise in response to infection, inflammation, malignancy, etc.
Best wishes,
My son is 10 and has C282Y homozygosity. He has been sleeping 15-18 hours a day for 2 months now. His iron levels are elevated. Ferritin is 376 and iron saturation is 84%. Doctors can’t find anything else wrong with him but don’t want to do a phlebotomy because it could stunt his growth. We are just concerned that what will stunt is growth is sleeping so much and not really getting enough to eat!
I’ve asked around and have found a few other kids with this same issue. It’s split down the middle…some do treatments and their kids feel better. The others don’t do treatments but monitor (they seemed to have lower levels than our son’s though).
Thoughts? We are at a loss and need to know if we should keep seeking other medical attention.
Hi Brittany,
Thank you for your message.
I would initially suggest checking his thyroid functions in view of the fatigue and sleepiness.
It’s also worth trying to avoid foods rich in iron like liver, spinaches, etc.
Limiting vitamin C may also decrease iron absorption.
If the above measures are not enough to control his serum iron and % saturation I would suggest phlebotomy based on his age and body weight to avoid organ damage.
Best wishes,
Hello Dr. Abdou,
I am a relatively healthy, normal BMI female (28yo) and recently had labs drawn. My H/H was very slightly elevated at 15.8/45.2 and ferritin was 232 (I’ve never had a previous one drawn). My liver panel, thyroid panel, hormone panels, were completely normal. I barely eat iron-containing foods, don’t take any vitamins/supplements, and rarely drink alcohol. I don’t think it’s high enough to consider for hemochromatosis but was wondering what if anything I should follow up about (and with who). And what if any labs should be repeat drawn.
Thank you for your time!
Hi Diana,
Thank you for your comment.
The management depends on the genetic mutation to the HFE gene and the fasting serum iron, transferrin saturation (TS), and ferritin.
In women of childbearing age, periods help to delay the build-up of serum iron levels.
I would suggest keeping your TS around a 30% mark and ferritin <100 by phlebotomy at 2-3 monthly intervals and regular follow up with a Hematologist or Gastroenterologist.
Best wishes,
Thank you for this article. I was informative beyond anything I have read before and very concise. Thank you again.
Hi Heather,
Thank you for your comment.
I’m pleased to hear that.
BW,
Hi! I am a female 40 and healthy as I thought until I got my lab results and I am trying to interpret them.. Iron & TIBC : Iron Bind Cap 406; UIBC 374; Iron 32; Iron saturation 8. My CBC is normal; B12 is 267 and folate 12.4. I am very concerned about Iron saturation of 8. What other labs should I do and how bad is my test result? Thank you!
Hi,
The most common cause of iron depletion in females of child-bearing age is blood loss e.g. heavy periods.
I would suggest checking your serum Ferritin.
You can also read more about that through this link:
BW,
Hi Dr l am a 57yr female diagnosed with fibromyalgia in 2019 but was only just told that my iron and b12 has been high since that time (recent test ferritin 107 and b12 1804). My hair is coming out by the handful and I’ve also just developed gerd. Dr says my liver function is fine and is no longer trying to find a reason for the elevated levels. Can you recommend any tests that l should get done privately?
Thank you in advance
Hi Margaret,
Thank you for your comment.
Ferritin is an acute-phase reactant protein that could rise in response to inflammation, infection, or malignancy.
Alcohol can also affect your serum ferritin level.
Your ferritin is not high, but I would suggest initially checking your fasting serum iron and iron saturation (transferrin saturation).
If your fasting serum iron and iron saturation are normal this would be reassuring but if high I would advise checking your Haemochromatosis genetic test (HFE).
BW,
Ferritin is 197 sorry!
Hi,
Please see my previous reply.
BW,
Hello I am a 35 year old who accidentally found hemochromatosis on my annual.
Ferritin 1357
saturation 61
total 151
IBC 246
CBC’s are normal
liver enzymes are normal
recently had a liver ultrasound and pending my HFE results (I am so anxious)
if not genetic my theory is that this is from my trauma 2 yrs ago car accident
femur fracture metal implant. I refused a blood transfusion to which I was infused with IV iron dextran and iron sucrose plus two rounds of erythropoietin
I suspect that the implant is causing a systemic inflammation as my ANA is positive
and my thyroid is also High at 400 in antibodies
Im going crazy, joints do hurt especially my hands.
please give me some advise as to how to proceed next.
my parents have not had the HFE test but their ferritin is normal.
Hi Liliana,
Thanks for your comment.
Until you have your Hemochromatosis genetic test result I would suggest to check your ESR, CRP, and the Rheumatoid Factor. I don’t think the iron therapy you had received 2 years ago is still in your system and causing your iron overload!
Systemic inflammation can cause your serum ferritin to rise but not the iron saturation.
BW,
Okay HFE results are back: NEGATIVE
CRP normal range
ESR normal range
RF normal range
Thyroid peroxidase antibodies 370
ANA titer is 1:80
ANA screen positive
pattern; nuclear dense fine speckled
Hello- is serum iron, ferritin, etc. ALWAYS elevated in Hemochromatosis? Is a diagnosis ever made based on positive gene markers and symptoms- but normal serum iron studies? Thanks
Hi Elizabeth,
Thanks for your comment.
Serum iron, transferrin saturation, and ferritin are commonly raised although not always. For example, serum iron may be normal in young women in spite of having hemochromatosis because the menstrual blood loss may mask the disease.
Approximately 87% of individuals of European origin with type 1 HFE-related hereditary hemochromatosis are either homozygotes for the p.(Cys282Tyr) pathogenic variant or compound heterozygotes for p.(Cys282Tyr) and the p.(His63Asp) disease associated polymorphism in the HFE gene.
BW,
Hi, I am a 67 year old female. Just recently was tested and found out that I am a carrier for the c282y gene. My results are: ferritin level 462, serrum iron 97, TIBC 253, TRANS ferrin iron is 38%. All my liver function tests are normal and all other blood tests are normal. My doctor said she will retest in a year. What is your opinion?
Hi Linda,
Thanks for your comment.
You’re at mild-moderate risk for iron overload and I would suggest to have infrequent venesections or to donate blood every 3 months or so aiming to keep your Ferritin <100 and your transferrin saturation around the 30% mark.
You should also screen your children.
BW,
Hi, I am 47 year old, male with tiredness, various aches and pains in joints and abdomen. I was recently diagnosed with iron overload (serum ferritin > 1400) with alanine aminotransferase =85 iu/litre. I have an Ultrasound booked in 10 days time, and am waiting for a genetic test. A lot of Venesection will be required i supposed. I wonder what the urgency is for starting venesection. Should I push to start this sooner than the Ultrasound is scheduled or is there likely to be a reason to wait until after the Ultrasound findings? How might the US findings affect my treatment program?
Many Thanks, J
Hi James,
Thank you for your message.
Ferritin is an acute-phase reactant protein that rises in a variety of conditions like infection, inflammation, liver disease, malignancy, and hemochromatosis.
If your fasting serum iron and transferrin saturation are normal I would suggest waiting until you have your hemochromatosis genetic test (HFE) and your liver ultrasound scan results.
BW,
Hello Dr Abdou,
I am a 63 year old female, relatively healthy until Dec 2019. At that time I had a lengthy period of abcess healing and unusual blood results since then. Family physician supsected hemochromatosis. Referral to hematologist and negative Blood test (assume genetic) for polycythemia and related myoprolif disorders. Ultrasound showed mild fatty liver. Hematologist ruled out Hemochromatosis based on blood work but no genetic testing. Dermatologist seen for red painful face rash, red itchy palms with slight contracture in one finger – rosacea and suspected Duprutrens contracture. Joint pain and red painful palms with lumps led me to a rheumatogolist who performed tests to rule out malignancies related to paraneoplastic palmar fasciitis – imaging was negative for lungs and organs with blood work showing elevated abnormalities continuing.
Hemoglobin elevated 169-175
Ferritin has been high since 2018 314-489
Hematocrit high .449 to .487
MCH slightly elevated
Ferritin high 314 to 489
ALT fluctuates between high and slightly elevated 38-48
AST fluctuates between 49 – 31
C reactive at 6.8 in Jun 2021
Rheumatologist considering Erythromelalagia and consult with hematologist. Received a cortisone treatment for left hand with the contracture type condition.
Question: could there be multiple disorders at play or should hemachromatosis still be considered for genetic testing? This journey has been a roller coaster……..looking for direction
Thank you
Hi Heather,
Thank you for your comment.
Your persistently raised ferritin is likely reactive to an underlying inflammation/infection or to your fatty liver.
I would suggest checking your fasting serum iron and transferrin saturation, if they are normal it is very unlikely that you have hemochromatosis
BW,
Hello Dr, I’d appreciate your advice with my labs. My whole life I’ve been anemic and iron deficient (I’ve been on iron supplements since I was a kid but nothing really seemed to help). However today my fasting serum iron level came back 195! Saturation was 52% & Total iron binding capacity was 374. Ferritin is low at 19. Hemoglobin was 9.4 (when previously it was 11.7). Hematocrit =28.9, Erythrocytes = 3.06, MCV = 94.4, RBC Distribution width = 27.4. I haven’t taken any iron medication for 2 months! What could cause this? I’m a 30 year old female not on oral contraceptives. My thyroid has been removed due to papillary thyroid cancer so I take levothyroxine. My TSH is 58 and T4 1 (my doctor has increased my dose). I have deficiencies in vitamin A. Vitamin C, E, K, D, & B12 are low normal range. Potassium, calcium, & magnesium are all normal. Liver was tested a month ago – values were all normal (they were – Billirubin = 0.3, Bilirubin total = 1, AST = 31, ALT = 11, Alkaline Phosphatase = 96, Albumin = 4.4, & Protein = 6.5). I have 2 genetic disorders – Factor V Leiden (d- dimer was checked to rule out clots – was normal at 233) & Ehler Danlos Syndrome Hyper mobility type 3. Thank you for any help. My doctors are stuck! (They are thinking a bleeding ulcer for the drop in hemoglobin but can explain the high iron – especially since I am not taking supplements, eating iron rich foods or cooking in a cast iron pan).
Hi Colleen C,
Thank you for your comment.
I would suggest a blood film, reticulocyte count, direct Coombs test, and serum immunoglobulins/protein electrophoresis to further investigate your anemia.
You haven’t mentioned if you had any previous bone marrow biopsy and GI work-up or not?
Ferritin reflects mainly the stored iron. Iron stores take some time to build up.
BW
I was wondering how the genes work with hemochromatosis, there are 6 children in my family and I have one copy of C282Y and one copy of H63D, my brother has one copy of H63D and one of my sisters has one copy of C282Y, one of my other sisters was told she has hemochromatosis but doesn’t know what her gene is she is going to find out. I have 2 sisters that haven’t been tested yet, my question is what did my parents have they are deceased so can’t be tested. Are each a carrier of the different variant or did one have one copy of each variant.
Hi Patricia,
Thank you for your comment.
You are compound C282Y/H63D heterozygous and you may develop iron overload but you are not classified as Type 1 hemochromatosis who are usually homozygous for the C282Y mutation in the HFE gene.
It sounds that one of your parents was a carrier (heterozygous) for the p.(His63Asp) disease associated polymorphism and the other was a carrier for the p.(Cys282Tyr) pathogenic variant.
Generally speaking, Type I hemochromatosis is caused by defects (mutations) in the HFE gene.
HFE has many purposes, but one important role is that it helps to control the amount of iron that is absorbed from food.
There are several known mutations in the HFE gene, but presently testing for only three is available: C282Y, H63D, and S65C.
BW,
Hello Dr. Abdou,
I very much appreciate the informative article
I can’t make sense of my labs and was wondering if you might have any suggestions or insight.
Total Iron 237
IBC 333
% Saturation 71
Ferritin 43
All liver numbers normal except Biliruben @ 1.7
ALK PHOS 39
AST 20
ALT 16
ALBUMIN 4.2
Prothrombin time normal
CRP 0.4
Thank you for your time
Eric
Hi Eric,
Thank you for your comment.
I would suggest checking your fasting serum iron and % saturation once again and if they are persistently high to have the hemochromatosis genetic test (HFE) to out rule hemochromatosis especially if you have a family history of the disease or you are a white Caucasian of Northern European descent.
BW,
Hello, I my 13 year old daughter has elevated serum iron (154 mcg/dL) and normal ferritin (42 ng/mL). Transferrin was normal 266mg/dL and TIBC was normal at 333mcg/dL. Iron sat was 46%. Her pediatrician was not sure what the significance of the elevated iron was with a normal ferritin. Do we need to worry about hemachromatosis for her or need additional testing. She was NOT fasting when labs were drawn (not sure if that could account for the elevated iron maybe?).
By the way, workup was started for 1 month of nausea (not pregnant, not anxious or depressed). Only other abnormality on labs was a pretty low vitamin D 25 OH (13), and total bilirubin of 0.8 (considered borderline elevated by this lab, other LFT’s were all low normal).
We are going to treat the low vitamin D, but I’m not sure if the hemachromatosis possibility is ruled in/out or if additional testing is needed based on these results. Thank you in advance!
Hi Albert,
Thank you for your comment.
A single elevated serum iron test result may not be significant.
However, I would suggest checking her fasting serum iron and % saturation (transferrin saturation) once again and if they are persistently high to have the hemochromatosis genetic test (HFE) to out rule hemochromatosis especially if she has a family history of the disease or she is a white Caucasian of Northern European descent.
Be sure she is not taking any iron or multivitamin pills.
BW,
My daughter has been suffering from general fatigue for years. She is 23 and recently had labs drawn which showed the following:
Hgb 14.3
Hct 42.4
Ferritin 63 ng/ml with normal range 12 to 122
Iron 144 ug/dl with normal range 37 to 145
Iron saturation 61% with normal range 20 to 55
TIBC 263 with normal range 228 to 428
She had taken a 23 and me test that showed an increased risk for hemochromatosis
Her NP did not seem concerned with her Iron values. She had been taking Vitamin C supplements. Her B12 was also 1800 but she was taking a B complex vitamin and a B12 supplement because she thought she had PMDD due to her fatigue. Her Vitamin D level was low as well.
Do these Iron studies indicate a need for further testing for hemochromatosis? Thanks in advance.
Hi Angela,
Thank you for taking the time to comment. Based on the information provided, it appears that your daughter has a high iron saturation and serum iron levels that are in the upper range of normal for their age group. This is somewhat unexpected given the monthly blood loss that typically occurs during menstruation, which can deplete iron stores.
To better understand the situation, I would recommend that your daughter’s fasting serum iron and iron saturation levels be checked once again. If these levels remain persistently high, it may be worth considering a hemochromatosis genetic test (HFE) to rule out any underlying genetic factors contributing to the elevated iron levels.
Thank you again for reaching out, and please don’t hesitate to let me know if you have any further questions or concerns.
Best regards,
Dr M Abdou
hi
Ferritin 470, 500, and 700. Every time I do an analysis, I find a variable percentage
But trasferrin is normal at 24%.
My iron test is normal
But liver enzymes GGT rise between 53 and 70 times when iron storage increases
CRP is negative
, normal ECR
, and plain NFS (CBC).
But the question is why are iron stocks rising? Is there an analysis that reveals the reason for its increase?
Thank you
Hi,
Thank you for your inquiry.
Based on the information you provided, it is possible that the elevated ferritin levels could be attributed to liver disease.
In order to further investigate this, I would recommend scheduling a liver ultrasound scan and consulting with a Hepatologist.
They will be able to provide more specialized insight and guidance on how to proceed.
BW,
Dear Dr. Abdou,
Thank you for the article. I commend you for explaining complex medical concepts in a way that is accessible to someone outside the field. Your informative piece not only expanded my understanding of hemochromatosis, but also highlighted the valuable contributions you make to the field. Thank you for your dedication to advancing knowledge and improving healthcare.
Best regards,
Mohab
Hi Mohab,
Thank you for taking the time to comment on my article about hemochromatosis.
I’m glad to hear that you found the information helpful.
Best regards,
Dr M Abdou
Hello Dr Abdou,
60 y.o. male in good health.
I consistently have a Saturation Percentage of 50 while my ferritin is in the 40s.
What would explain this type of divergence? Is there a way to lower sat percentage without lowering ferritin?
Thank you!
Hi Ronn,
Thank you for your comment.
I would suggest initially checking your fasting serum iron, liver function tests (including gamma GT), along with an ultrasound scan on your liver.
Best regards,
Dr M Abdou
Hi Dr Abdou, I’m a first year medical student currently doing an academic poster on Haemochromatosis. Do I have permission to use the images on this page? Also, if you have any links or interesting data in it’s role of causing hypogonadotropic hypogonadism then that would be perfect! Thanks
Hi Oli,
Thank you for your comment.
You are welcome to use the images included in my haemochromatosis article, provided that you include the link to the article on your academic poster references.
Yes, hypogonadotropic hypogonadism is one of the classical complications of hemochromatosis.
You can read more through this link: Hypogonadotropic hypogonadism in men with hereditary hemochromatosis
Best wishes,
Dr. M Abdou