Erythropoiesis Stimulating Medicines
The kidney cells that make erythropoietin are sensitive to low oxygen levels in the blood that travels through the kidney. These cells make and release erythropoietin when the oxygen level is too low. A low oxygen level in the blood may be seen in lung and heart diseases and may indicate a diminished number of red blood cells (anemia), or hemoglobin molecules that carry oxygen through the body.
Red blood cells contain a substance called hemoglobin, which, as well as making blood red, carries oxygen around the body. If the body does not receive enough oxygen because too few red blood cells are circulating, this is called anemia. There are various symptoms of anemia including tiredness, weakness, and lack of energy.
ESA medicines are usually prescribed for patients with chronic kidney disease (CKD). Anemia is a common side effect of CKD because the kidneys cannot release enough erythropoietin. Other uses of ESA medicines may include treatment of anemia related to the medication AZT (used to treat AIDS), anemia caused by chemotherapy, anemia caused by dysfunctional bone marrow (myelodysplasia), and anemia associated with cancer. EPO may also be prescribed for low birth weight premature babies who have anemia.
The US Food and Drug Administration (FDA) advises clinicians to consider starting ESA treatment for patients with CKD when the hemoglobin level is less than 10 g/dL, but does not define how far below 10 g/dL would be an appropriate threshold for initiating ESA treatment in an individual patient.
What is recombinant erythropoietin?
In cases where transfusions are not an option—for example, when the patient cannot have or refuses, a transfusion—it may be necessary to give the patient recombinant erythropoietin. Recombinant erythropoietin is a man-made version of natural erythropoietin. It is produced by cloning the gene for erythropoietin.
Recombinant erythropoietin drugs are known as erythropoietin-stimulating agents (ESAs). These drugs are given by injection and work by stimulating the production of more red blood cells. These cells are then released from the bone marrow into the bloodstream.
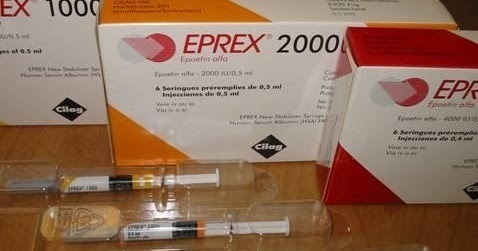
There are two ESAs on the U.S. market: epoetin alfa (Procrit®, Epogen®, Eprex®), and darbepoetin alfa (Aranesp®).
What are the side effects of ESAs?
The side effects that occur most often with ESA use include:
- High blood pressure
- Fever
- Dizziness
- Nausea
- Pain at the site of the injection.
Blood pressure should always be closely monitored in patients administered with such agents.
ESA resistance:
The working definition of ESA resistance is the requirement for greater than 150 units/kg of ESA at least 3 times per week or the sudden response refractoriness to a previous stable maintenance dose, such that hemoglobin levels fall below target levels.
The most common cause of ESA resistance is iron deficiency. Therefore, it is imperative that iron stores are adequate during ESA treatment. The second most common cause of ESA resistance is a chronic infection/inflammatory state, and such resistance is attributed to inflammatory cytokines (eg, IL-1).
Other less common causes of ESA resistance include hyperparathyroidism (the mechanism appears to be related to bone marrow fibrosis), as well as severe malnutrition.
Safety issues:
There are several safety issues with erythropoiesis-stimulating medicines:
- ESAs increase the risk of venous thromboembolism (blood clots in the veins). A blood clot can break away from one location and travel to the lung (pulmonary embolism), where it can block circulation. Symptoms of blood clots include chest pain, shortness of breath, pain in the legs, and sudden numbness or weakness in the face, arm, or leg.
- ESAs can cause hemoglobin to rise too high, which puts the patient at higher risk for heart attack, stroke, heart failure, and death.
- In patients who have cancer, ESAs may cause the tumor to grow. If ESAs are used for these patients, they are usually stopped after the patient’s chemotherapy is finished.
- The health care provider will keep an eye on the patient’s blood cell counts to make sure they do not put him or her at a higher risk. The dosing may change, depending on the patient’s needs.
Patients who have the following conditions need to consult with their health care provider if erythropoiesis-stimulating medicines are being considered as part of the treatment plan:
- Heart disease
- Uncontrolled high blood pressure
- Porphyria (a group of diseases that are caused by enzyme deficiencies)
- Seizures
- An allergy to epoetin alfa or any other part of this medicine
In addition, women who are pregnant, planning to become pregnant, or breastfeeding should consult with their health care provider before taking an ESA.
References:
Siamak N. Nabili, MD, MPH. Erythropoietin (EPO, The EPO Test). https://www.medicinenet.com/erythropoietin/article.htm#erythropoietin_epo_definition_and_facts
Erythropoietin-Stimulating Agents. Cleveland Clinic https://my.clevelandclinic.org/health/drugs/14573-erythropoietin-stimulating-agents
National Kidney Foundation. Anemia and Chronic Kidney Disease. Accessed 6/15/2018.
Edgar V Lerma, MD. Anemia of Chronic Disease and Renal Failure: Overview, Mechanism of Anemia of Chronic Disease, Prevalence of Anemia of Chronic Disease and CKD https://emedicine.medscape.com/article/1389854-overview
FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. U.S. Food & Drug Administration. Available at https://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. June 24, 2011; Accessed: November 25, 2018.