CAR T-Cell Therapy
The mechanism of action of CAR T-cell therapy:
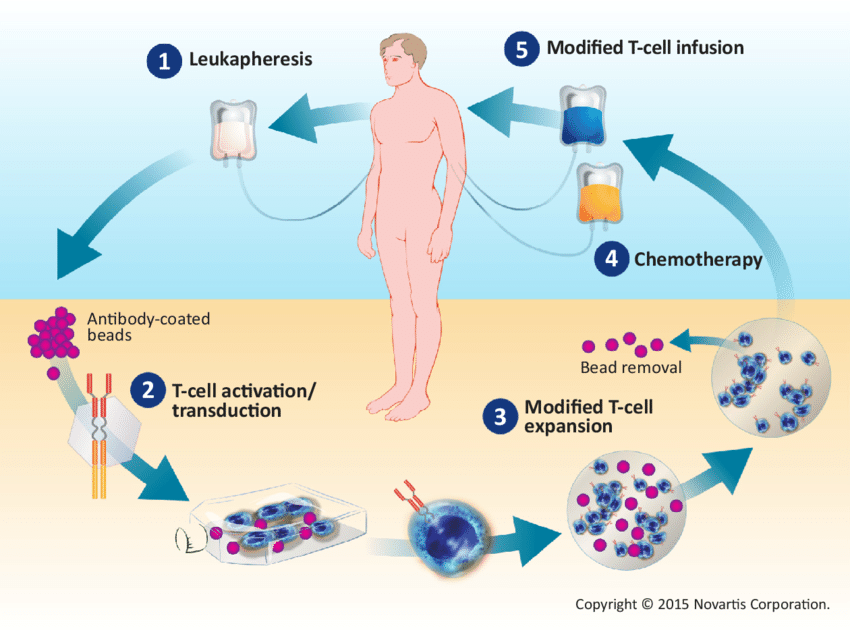
A sample of a patient’s T cells is collected from the blood and then modified to produce special structures called chimeric antigen receptors (CARs) on their surface. When these CAR T cells are reinfused into the patient, the new receptors enable them to latch onto a specific antigen on the patient’s tumor cells and kill them.
T cells:
To understand CAR T-cell therapy, it helps to understand what T cells do.
White blood cells called lymphocytes play an important part in fighting infection and diseases, including cancer. There are different types of lymphocytes. T cells are one type.
T cells move around the body to find and destroy defective cells. When you come into contact with a new infection or disease, the body makes T cells to fight that specific infection or disease. It then keeps some in reserve so that if you come across the infection again your body can recognize it and attack it immediately.
CAR T-cell therapy:
T cells are good at fighting infection. However, it can be difficult for them to tell the difference between a cancer cell and a normal cell. So the cancer cells can hide away and not be recognized. Scientists are trying to find ways to get T cells to recognize cancer cells. One possible way to do this might be CAR T-cell therapy.
CAR (Chimeric Antigen Receptor) T-cell therapy is a cancer treatment that is making headlines. In fact, it is now on the road to being one of the most promising treatments in oncology. CAR T-cell therapy is a form of immunotherapy that uses specially altered T cells to fight cancer. A sample of a patient’s T cells is collected from the blood and then modified to produce special structures called chimeric antigen receptors (CARs) on their surface. When these CAR T cells are reinfused into the patient, the new receptors enable them to latch onto a specific antigen on the patient’s tumor cells and kill them.
CAR T-cell therapy was discovered in 1989 but was not put into high gear until recently. It is already changing how patients with blood cancers who have not been responsive to previous therapy are being treated. Although the technology was described almost three decades ago, clinical implementation did not occur until recently.
The 2019 annual meeting of the American Society of Haematology (ASH), held on 6-10 December, was focused on what was described as “the next big thing” in blood cancers — CAR T-cell therapy, a single-infusion treatment which relies on a patient’s own immune system cells to battle cancer.
CAR T-cell therapy has been hailed as a major advance in hematology — in 2018, the American Society of Clinical Oncology (ASCO) named CAR T-cell immunotherapy as its ‘Advance of the Year’. The therapy has shown significant achievements in certain types of blood cancers, and efforts are now also underway to turn CAR T-cells against multiple types of cancer, including solid tumors.
Turning the body’s immune system against cancer is not a new idea, but what makes CAR T-cell therapy revolutionary is that the treatment involves extracting immune cells from a patient, genetically altering them to better recognize and battle a particular cancer, and then inserting them back into the body. CAR T-cell treatment has been described as cell therapy, gene therapy, and immunotherapy all rolled into one.
After a patient’s cells are withdrawn, they are primed in a laboratory to recognize and attack cancer through a genetic modification process that causes T-cells to express a receptor on their surface. The special receptor is known as the chimeric antigen receptor or CAR. The cells’ receptor is engineered to bind to a protein on cancer cells known as CD19.
CAR T-cell therapy represents hope for patients with B-cell lymphoma who have failed all other treatments. In the EU, two CAR T-cell therapies are licensed for use: Kymriah (tisagenlecleucel), and Yescarta (axicabtagene ciloleucel).
Kymriah is indicated for the treatment of pediatric and young adult patients (up to 25 years of age) with B-cell acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse, and in adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy.
Yescarta is indicated for the treatment of adult patients with relapsed or refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL), after two or more lines of systemic therapy.
Innovative CAR T-cell therapy represents hope for blood cancer patients who have exhausted all other treatment options, but only if it is accessible. In clinical trials, CAR T-cell therapy has shown excellent remission rates in severe forms of blood cancer. This is particularly impressive, considering most CAR T-cell clinical trials recruit cancer patients who have not responded to many if not all other available treatments.
However, CAR T-cell therapy requires time — it takes some weeks in the lab to administer the treatment working with the patient’s extracted blood cells — and it is expensive.
Who is eligible to receive CAR T-cell therapy?
Currently, CAR T-cell therapy is FDA-approved as a standard of care for some forms of aggressive, relapsed, or refractory non-Hodgkin lymphoma including diffuse large B cell lymphoma, primary mediastinal B-cell lymphoma, high-grade B-cell lymphoma, transformed follicular lymphoma, and mantle cell lymphoma. In addition, CAR T-cell therapy is approved for patients with relapsed or refractory acute lymphoblastic leukemia up to age 25.
There are also many clinical trials of CAR T-cell therapy for other types of blood cancer and solid tumors.
CAR T-Cell Therapy Process:
The treatment process involves:
Evaluation: Patients undergo a series of tests and screenings to determine if CAR T-cell therapy is an appropriate option.
Collection: T cells are collected from patients via apheresis, a process that withdraws blood from the body and removes one or more blood components (in this case, T cells). The remaining blood is then returned to the body.

A patient’s immune system T cells are collected through a process known as leukapheresis. Blood from the patient’s arm is channeled to an apheresis machine, which separates out T cells and other cells and returns the remaining blood components to the other arm. When the process is complete, the collection bag holds 10-20 billion cells, about half of which are T cells.
Engineering: The T cells are sent to a laboratory where they are genetically engineered to express chimeric antigen receptors (CARs) on their surface. CARs are proteins that allow the T cells to recognize an antigen on targeted tumor cells.
Multiplication: The genetically modified T cells are “expanded” by growing cells in the laboratory until there are millions of them. This process can take a few weeks. When there are enough CAR T cells, they are frozen and sent to the hospital or center where the patient is being treated.
Conditioning Therapy: Prior to infusion of the CAR T cells, patients may receive chemotherapy for their cancer. This helps to create space in your immune system for the infused CAR T cells to expand and proliferate.
Infusion: Soon after chemotherapy, patients are admitted to the hospital and the CAR T cells are re-infused in a process similar to a blood transfusion. This is a one-time infusion, although patients may remain in the hospital for several weeks to monitor response to treatment, overall condition, and side effects.
Recovery: Patients who receive CAR T-cell therapy have a risk/recovery period of approximately 2-3 months. During this period, patients will be evaluated for side effects and treatment response. It is not uncommon for patients to be re-admitted to the hospital during this period to manage complications. During the first 30 days after discharge from the hospital, patients need to remain close to their center for regular follow-up care.
What does the recovery process entail?
Recovery can take time as the immune system recovers. The acute recovery period is typically 30 days after the CAR T-cell infusion. During this time, patients must remain within two hours of their cancer center and must have a caregiver with them at all times to monitor for signs of fever, infection, and neurologic difficulties. Most patients feel tired and don’t have much appetite during this period.
What are the possible side effects of CAR T-cell therapy?
Although most patients do not experience the common side effects associated with chemotherapy such as hair loss, nausea, and vomiting, there are risks of significant side effects with CAR T-cell therapy. Patients are admitted to the hospital for several weeks so the care team can monitor response to the treatment, and manage reactions to this therapy. The complications are generally temporary and resolve with treatment. The care team is specially trained to identify and manage these side effects.
Possible side effects from CAR T-cell therapy include:
- Cytokine release syndrome: CAR T cells can initiate a massive release of substances called cytokines, which triggers an inflammatory condition known as cytokine release syndrome (CRS). Symptoms may be flu-like, with a high fever and/or chills; low blood pressure; difficulty breathing; or confusion. These symptoms can be mild or severe.
- Neurologic difficulties: Patients may also experience confusion, difficulty understanding language and speaking, or stupor.
- Infections: Infections account for nearly half of all deaths not caused by disease relapse among patients with advanced blood cancers after treatment with chimeric antigen receptor (CAR) T-cell therapy, a new meta-analysis of clinical trials and real-world studies shows. When studies specified the pathogen linked to nonrelapse mortality, COVID-19 accounted for more than half of these deaths, fungal infections accounted for 18%, and bacterial pathogens accounted for 20.2%. The remaining deaths were attributed to unspecified viral causes or infection. In terms of treatment, approaches for prophylaxis are acyclovir (an antiviral to prevent shingles) and Co-trimoxazole or similar medicine to prevent opportunistic infection, particularly Pneumocystis jirovecii pneumonia. Studies also strongly recommend that these patients receive their vaccinations before the start of CAR T therapy, if possible. It’s also important, when possible, that they do not receive anti-CD20 antibodies, such as rituximab, for 6-12 months before vaccination, as these treatments can also cause B-cell suppression that may last long after treatment is given.
CAR T-cell therapy efficacy and cost:
New research on CAR T-cell therapy was presented at the 61st ASH annual meeting. ASH Secretary Dr. Robert Brodsky, speaking to the media at the conference, described why CAR T-cells have “captured the imagination of physicians and patients alike” for their “incredible efficacy” in treating B-cell malignancies like ALL and non-Hodgkin’s lymphomas.
Dr. Brodsky also discussed some of the issues surrounding CAR T-cell therapy, including the time and expense that it takes to generate a CAR T-cell product for a specific patient.
It can take two weeks to make such a product, as the process has several key steps. Blood taken from the patient needs to be prepared, packaged, and sent to a specialized manufacturing facility, where the cells are genetically engineered and expanded in culture, and then the product — prepared specifically for that one individual — is transported back and administered to the patient.
“Only about two-thirds of patients enrolled in CAR T-cell trials will actually get the infusion because often, the disease will progress during the time that it takes to make a successful product,” he said.
However, new real-world data presented at the ASH conference suggests that CAR T-cell therapy may actually be cost-effective, as it may lower other expenses related to lymphoma.
When used in a population of older adults with non-Hodgkin’s lymphoma, these new data show that CAR T-cell therapy cut related expenditures compared with healthcare costs prior to receiving this treatment. “CAR T-cell therapy was associated with fewer hospitalizations, shorter time in the hospital, fewer emergency department visits, and lower total healthcare costs,” said lead study author Dr. Karl Kilgore, Ph.D., of Avalere Health in Washington DC, US.
In the study, Dr. Kilgore and colleagues evaluated the demographic and clinical characteristics of Medicare patients who received CAR T-cell therapy (axicabtagene ciloleucel or tisagenlecleucel) and then compared healthcare utilization, costs, and outcomes pre- and post-CAR T-cell therapy.
“The goal of this study was to look at the real use of CAR-T cell therapy and real-world data on the use of these therapies,” said Dr. Kilgore. “And to look at healthcare utilization.”
Patients were included in the study if they had been diagnosed with lymphoma and received CAR T-cell therapy between 1 October 2017, and 30 September 2018. A total of 177 patients met all of the inclusion criteria and were included in the analysis.
The average age was 70 years; more than half (58.8 percent) were male, and they were primarily white (87.6 percent). Nearly all patients (91.5 percent) had a primary diagnosis of DLBCL, as well as multiple comorbidities, with 74.6 percent having a Charlson Comorbidity Index score ≥ 3. Fewer than 5 percent of patients had undergone a previous autologous stem cell transplant, and 51 percent had one or more comorbidities that would have disqualified them from participating in CAR T-cell clinical trials (ie, renal failure, heart failure, a recent history of DVT/PE).
Just over half of the participants (52 percent) had been treated with intravenous chemotherapy in the six months before receiving CAR T-cell therapy, and 60 percent received outpatient lymphodepletion.
During their index episode of care for CAR T-cell infusion, the patients spent a median of 16 days (interquartile range, 10) in the hospital, and 45.5 percent required ICU care after infusion. In the six months before CAR T-cell therapy (pre-index), 51.2 percent had been hospitalized at least once, and almost 20 percent had three or more periods of hospitalization. Of that group, 27.1 percent were readmitted during the post-index period. Among patients who required hospitalization, the median length of stay pre-index was seven days, and five days post-index. The number of ED visits was also lower in the post- vs pre-index (15.8 percent vs 29.9 percent).
“Patients spent 17 percent less time in the hospital six months after CAR T-cell therapy than before,” Dr. Kilgore said.
As for cost, the median total healthcare costs during the pre-index period were US$51,999 (mean, 58,820; standard deviation (SD), 45,701) and US$14,014 post-index (mean, 23,738; SD, 29,698). This extrapolates into US$9,749 pre- vs $7,121 post-index for each patient per month, which is a 27 per cent decrease in expenditures.
Dr. Kilgore believes that the key takeaway from this study is that the majority of participants — who were older and sicker than many enrolled in CAR T-cell clinical trials — did quite well.
“Diffuse large B-cell lymphoma is a disease that usually causes people to die quickly if they are refractory to multiple lines of therapy, so the six-month survival of 75 percent in this patient population is impressive,” he said. “A large proportion of these patients are likely to have died had they not received CAR T-cell therapy.”
Secondary Cancers From CAR T-Cell Therapies:
The US Food and Drug Administration (FDA) is investigating whether chimeric antigen receptor (CAR) T-cell immunotherapies can cause secondary blood cancers.
Secondary cancers are a known risk for this class of immunotherapies, known as B cell maturation antigen (BCMA)–directed or CD19-directed autologous CAR T-cell therapies, and are included in the prescribing information for these drugs. However, the FDA has received 19 reports of secondary cancers, including CAR-positive lymphoma, since 2017, when the first CAR T-cell treatments were approved, according to Endpoint News.
Most of these reports came from the FDA’s postmarketing adverse event system and others from clinical trial data.
Although the overall benefits of these products continue to outweigh their potential risks, “FDA is investigating the identified risk of T cell malignancy with serious outcomes, including hospitalization and death, and is evaluating the need for regulatory action,” the agency said in a press release.
Currently approved products in this class include idecabtagene vicleucel (Abecma), lisocabtagene maraleucel (Breyanzi), ciltacabtagene autoleucel (Carvykti), tisagenlecleucel (Kymriah), brexucabtagene autoleucel (Tecartus), and axicabtagene ciloleucel (Yescarta).
Patients and clinical trial participants receiving treatment with these products should be monitored life-long for new malignancies,” the FDA added.
Suspected adverse events, including T-cell cancers, should be reported by contacting the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Summary:
CAR-T cell therapy is a type of immunotherapy that uses specially altered T cells to fight cancer. The collected T cells are genetically treated in the lab to produce special receptors called chimeric antigen receptors (CARs) that can recognize and attack cancer cells. CAR-T cell therapy is an individualized cell-based technique that involves removing some of the patient’s own white blood cells, including T cells. The treatment tends to be effective, but it also carries a risk of serious side effects.
References:
Sandra Ryan, Larry Bacon. What you need to know about CAR T-cell therapy https://www.medicalindependent.ie/what-you-need-to-know-about-car-t-cell-therapy/
CAR T-Cell Therapy for Cancer: What’s New on the Road to Cure? https://thedoctorweighsin.com/car-t-cell-therapy-cancer/
Ranveer Tanwar. For The First Time in Italy, Treatment Has Been Done Using Car T-cells Against Multiple Myeloma. https://www.sinceindependence.com/for-the-first-time-in-italy-treatment-has-been-done-using-car-t-cells-against-multiple-myeloma/
Building blocks for institutional preparation of CTL019 delivery – Scientific Figure on ResearchGate. https://www.researchgate.net/figure/Mechanism-of-action-of-CAR-T-cell-therapy-Patients-T-cells-are-collected-by_fig1_318693671 [accessed 1 Dec, 2020]
Frequently Asked Questions About CAR T-Cell Therapy – Dana-Farber Cancer Institute | Boston, MA https://www.dana-farber.org/cellular-therapies-program/car-t-cell-therapy/faq-about-car-t-cell-therapy/
Giavridis, T., van der Stegen, S.J.C., Eyquem, J. et al. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 24, 731–738 (2018). https://doi.org/10.1038/s41591-018-0041-7
CAR T-cell therapy. https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/immunotherapy/types/CAR-T-cell-therapy
CAR T cell therapy explained: Cancer types, success rate, and more. https://www.medicalnewstoday.com/articles/car-t-cell-therapy.
CAR T-cell Therapy and Its Side Effects – American Cancer Society. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/car-t-cell1.html.
About CAR-T cell therapy – Mayo Clinic. https://www.mayoclinic.org/departments-centers/car-t-cell-therapy-program/sections/gnc-20405215.
CAR T Cells for Solid Tumors | Center for Cancer Research. https://ccr.cancer.gov/news/milestones-2022/car-t-cells-for-solid-tumors.
CAR T-cell therapy | Cancer Research UK. https://www.cancerresearchuk.org/about-cancer/treatment/immunotherapy/types/CAR-T-cell-therapy.
Sharon Worcester. FDA Investigates Secondary Cancers From CAR T-Cell Therapies. https://www.medscape.com/viewarticle/998863
Neil Osterweil. Infection Main Cause of Nonrelapse Deaths With CAR T-Cell Therapy https://www.medscape.com/viewarticle/infection-main-cause-nonrelapse-deaths-car-t-cell-therapy-2023a1000w0c










Hello dear my name is kwestan Ahmed I live in Iraq/ Slemaniya. Recently I have diagnosed with breast cancer and honestly I am not satisfied with the chemotherapy that they use here. Iam asking if it is possible for me to get the newest therapy (T cell), even if I have to participate in a research to use the therapy. Also what is your advise for me as a cancer Patient.
Thanks
Hi Kwestan,
I wish you a speedy recovery and to get well soon.
The current standard of care for the treatment of breast cancer is surgery, radiotherapy, chemotherapy, monoclonal antibodies, hormonal therapy, and immunotherapy.
Although there are some clinical trials investigating the efficacy of CAR-T cell therapy in refractory/relapsing cases of breast cancer, it is not a substitute for standard treatment. Currently, CAR T-cell therapy is FDA approved as a standard of care for:
*Some forms of aggressive, relapsed or refractory non-Hodgkin lymphoma including diffuse large B cell lymphoma, primary mediastinal B-cell lymphoma, high-grade B-cell lymphoma, transformed follicular lymphoma, mantle cell lymphoma
*Relapsed or refractory follicular lymphoma
*Relapsed or refractory multiple myeloma
*Relapsed or refractory acute lymphoblastic leukemia
There are also many clinical trials of CAR T-cell therapy for other types of blood cancer and solid tumors.
I would therefore suggest proceeding with the standard treatment recommended for your breast cancer by your treating Oncologist.
BW,