Thrombocytopenia
Thrombocytopenia is a medical condition characterized by a lower-than-normal number of platelets in the blood. Platelets are crucial for blood clotting, so a low platelet count can lead to excessive bleeding and difficulty forming blood clots. The ICD-10 code for thrombocytopenia is D69. 6.
The hemostatic system comprises platelets, coagulation factors, and the endothelial cells lining the blood vessels. Platelets arise from the fragmentation of the cytoplasm of megakaryocytes in the bone marrow and circulate in the blood as disc-shaped anucleate particles for 7-10 days. About one-third are always transiently sequestered in the spleen. Platelets are eventually destroyed by apoptosis, a process independent of the spleen. The platelet count is normally 150,000 to 400,000/mm3.
Thrombopoietin helps control the number of circulating platelets by stimulating the bone marrow to produce megakaryocytes, which in turn shed platelets from their cytoplasm. Thrombopoietin is produced in the liver at a constant rate. Its circulating level is determined by the extent to which circulating platelets are cleared and possibly by bone marrow megakaryocytes.
Thrombocytopenia is defined as a platelet count of less than 150,000/mm3. With normal platelet function, thrombocytopenia is rarely the cause of bleeding unless the count is less than 50,000/mm3. Thrombocytopenia should always be confirmed by examination of a peripheral smear. It can be caused by decreased platelet production, increased destruction, sequestration, or a combination of these causes.
Causes of thrombocytopenia:
Platelet Underproduction:
The hallmark of platelet underproduction is decreased marrow megakaryocytes. Common causes include infections (including HIV), drugs (frequently chemotherapeutic agents or alcohol, but other medications in rare cases), radiotherapy, vitamin deficiency (e.g., folate, vitamin B12), or marrow infiltration by tumor, storage diseases, or marrow failure syndromes (e.g., aplastic anemia). In addition, the myelodysplastic syndromes are a frequently overlooked group of disorders associated with thrombocytopenia in older adults.
Management involves treating the underlying condition and supportive platelet transfusions if needed. More recently, two recombinant thrombopoietin (TPO) agents (recombinant human thrombopoietin, rhuTPO, and pegylated recombinant human megakaryocyte growth and development factor, PEG-rhuMGDF) have entered the clinical arena for the treatment of chemotherapy-induced thrombocytopenia.
A single dose of rhuTPO or PEG-rhuMGDF increases the platelet count after about 5 days, and the peak effect is observed about 10 to 12 days later; rhuTPO increases the nadir platelet count, reduces the duration of thrombocytopenia, and results in a decrease in platelet transfusion for patients receiving dose-intense chemotherapy for ovarian cancer. These agents remain the subjects of investigation, and recommendations for their use have not been defined.
Platelet Sequestration:
Hypersplenism from a variety of causes, including liver disease or malignancy, may result in platelet sequestration. Mild to moderate thrombocytopenia is caused by platelet sequestration when there is an associated mild reduction in neutrophil count and hemoglobin and with minimal impairment of hematopoiesis on bone marrow examination. If physical examination fails to detect splenomegaly, evaluation with ultrasonography or radionuclide imaging is recommended to document splenomegaly. 
 Management includes treatment of the underlying condition and platelet transfusion, as needed. Cytopenias secondary to hypersplenism are often not sufficiently severe to warrant treatment in the form of total or partial splenectomy, partial splenic embolization, or transjugular intrahepatic portosystemic shunting for congestive splenomegaly.
Management includes treatment of the underlying condition and platelet transfusion, as needed. Cytopenias secondary to hypersplenism are often not sufficiently severe to warrant treatment in the form of total or partial splenectomy, partial splenic embolization, or transjugular intrahepatic portosystemic shunting for congestive splenomegaly.
Increased Platelet Destruction:
The hallmark of increased platelet destruction is increased marrow megakaryocytes or, when available, high reticulated platelet count. Platelet destruction results from various immune conditions, including the following:
- Immune thrombocytopenic purpura (ITP)
- Thrombotic microangiopathies
- Post-transfusion purpura (PTP)
- Heparin-induced thrombocytopenia (HIT)
- Disseminated intravascular coagulation (DIC)
Immune Thrombocytopenic Purpura (ITP)
Prevalence
The incidence of ITP in a Danish study was 100 cases per 1,000,000 person-years, with 50% of cases occurring in the pediatric age group. It can be of adult or childhood onset. Adult onset is more likely to be chronic and insidious. Adult-onset ITP is more common in females than males (with a female-to-male ratio of 1.7 : 1), whereas childhood onset has equal gender distribution.
ITP is subdivided into chronic or acute, with the latter being of 6 months or less in duration.
Causes of Immune Thrombocytopenic Purpura
ITP can be primary or secondary. Causes of secondary ITP include systemic lupus erythematosus, antiphospholipid antibody syndrome, immunoglobulin A (IgA) deficiency, common variable hypogammaglobulinemia, lymphoproliferative disorders (e.g., chronic lymphocytic leukemia, lymphomas), viral (e.g., HIV, hepatitis C), or drug-induced—many drugs have been linked to thrombocytopenia, but those known to be associated with immune thrombocytopenia are heparin and quinidine. Patients with drug-induced ITP usually present within 1 to 2 weeks from the initiation of the offending drug with petechiae and a platelet count of less than 20,000/mm3. Recovery usually occurs 5 to 7 days after discontinuation of the offending agent but can occasionally be more prolonged.
Here we will focus on primary ITP. The guidelines are derived from recommendations of the consensus guideline of the American Society of Hematology.
Pathophysiology
The pathophysiology of primary ITP involves the formation of antiplatelet antibodies, frequently directed at platelet glycoproteins IIb/IIIA, IIb/IX, Ia/IIa, and V, or multiple platelet antigens.
Clinical Features
On history and physical examination, the absence of systemic symptoms is helpful in ruling out secondary causes. Evidence of platelet-type (mucosal) bleeding should be noted, and the absence of splenomegaly supports the diagnosis. Bleeding is often less pronounced than in cases of decreased production with similar platelet counts.
Diagnosis
The complete blood cell (CBC) count should be unremarkable except for thrombocytopenia or easy to account for anemia. The peripheral smear must confirm thrombocytopenia, and large immature platelets are often noted which is a reflection of the increased thrombopoietin-induced stimulation of bone marrow. 
Clumps of platelets on a peripheral smear prepared from ethylenediaminetetraacetic acid (EDTA)–anticoagulated blood are evidence of pseudothrombocytopenia. The diagnosis of this type of pseudothrombocytopenia is established if the platelet count is normal when repeated on a sample from heparin-anticoagulated or citrate-anticoagulated blood. 
Bone marrow aspiration and biopsy in patients with ITP demonstrates a normal-to-increased number of megakaryocytes in the absence of other significant abnormalities. The value of bone marrow evaluation for a diagnosis of ITP is unresolved, and more data are needed to establish clear guidelines. 
A bone marrow biopsy or aspirate is required when one of the following features is noted: patients older than 60 years, presence of atypical features (e.g., fatigue, fever, joint pain, macrocytosis, neutropenia) or before splenectomy in the patient whose diagnosis is not definitive.
Testing for antiplatelet antibodies is generally not recommended. Antiplatelet antibodies have a sensitivity of 49% to 66%, a specificity of 78% to 92%, and a positive predictive value of 80% to 83%. A negative test result does not rule out the diagnosis.
Treatment
First Presentation:
In the asymptomatic patients with a platelet count of less than 30,000/mm3 or in the symptomatic patient with a platelet count higher than 30,000 but less than 50,000/mm3, treatment with steroids such as prednisone, 1 to 1.5 mg/kg/day, has an expected response rate of 50% to 75%. A response is usually seen after days of treatment. Experts differ on the length of time needed before labeling the patient steroid-unresponsive and changing therapy. Accordingly, a trial of 1 to 3 weeks of a corticosteroid is considered an adequate therapeutic trial.
Intravenous immunoglobulin (IVIG), 1 g/kg/day for 2 to 3 days, is used to treat major bleeding, platelet counts of less than 5,000/mm3 despite 3 days of steroids, or extensive and progressive purpura. It is also the initial agent in patients with platelet counts of less than 50,000/mm3 with life-threatening bleeding. The response rate for IVIG is 80%. Disadvantages include cost, the low rate of long-term response, and risks of anaphylaxis (especially in patients with IgA deficiency), renal failure, or pulmonary failure.
Rho(D) immune globulin (RhoGAM) for Rh-positive patients, 75 μg/kg, is as effective but less toxic than steroids. Significant adverse effects of this treatment include a hemolytic anemia that rarely results in more than a 2-g/dL drop in the hemoglobin level. It is, however, more expensive than prednisone and affords a similar long-term remission (5% to 30%).
Splenectomy should be considered after 3 to 6 months if the patient continues to require 10 to 20 mg/day of prednisone to keep the platelet count higher than 30,000/mm3 or within 6 weeks of diagnosis in the patient with a platelet count of less than 10,000/mm3, despite treatment. Laparoscopic splenectomy is been increasingly used in high-volume centers and helps decrease the duration of hospitalization. Pneumococcal, meningococcal, and Haemophilus vaccination is indicated before splenectomy.
Thrombopoietin receptor agonists: recently, 2 thrombopoietin receptor agonists have been approved for the treatment of chronic refractory ITP. Eltrombopag (Promacta) is an oral nonpeptide thrombopoietin receptor agonist that interacts with the transmembrane domain of the thrombopoietin receptor and induces megakaryocyte proliferation and differentiation. It has been shown to increase the platelet count in refractory ITP and in thrombocytopenia associated with hepatitis C –induced cirrhosis. It will most likely also be effective in thrombocytopenia due to other causes, by stimulating megakaryocytes. Eltrombopag is given in doses of 25-75 mg daily. The adverse effects include hepatotoxicity, worsening of cataracts, and increased bone marrow reticulin fibers. In a phase III, randomized, double-blind, placebo-controlled trial, once-daily eltrombopag was shown to be effective for the management of thrombocytopenia in adults with chronic ITP.
Romiplostim (Nplate) is another thrombopoietin receptor agonist, consisting of human immunoglobulin Fc region covalently linked to a peptide sequence that binds to and activates the thrombopoietin receptor. Weekly subcutaneous doses of 1-7 μg/kg, romiplostim can increase the platelet count in chronic ITP. The adverse effects include bone marrow reticulin formation. In 2 parallel trials that assessed the long-term administration of romiplostim in 63 splenectomized and 62 nonsplenectomized patients with ITP, both the splenectomized and nonsplenectomized patients achieved durable platelet counts over a longer period with romiplostim than with placebo; patients receiving romiplostim were also more likely to reduce or discontinue concurrent other ITP therapy compared with patients in the placebo groups.
The responses to thrombopoietin receptor agonists take 10-15 days, and, hence, they are unlikely to replace steroids or intravenous immunoglobulins as initial therapy. Furthermore, relapses are common, necessitating long-term therapy. Thrombopoietin receptor agonists may help postpone or even prevent splenectomies. The advantages of thrombopoietin receptor agonist therapy should be weighed against the risk of marrow fibrosis seen in the limited long-term outcome data. There is also a theoretical possibility that these agents increase the risk of hematologic malignancies, as thrombopoietin receptor is present in hematopoietic stem cells. Currently, these agents are recommended for ITP patients whose conditions are refractory to previous treatments, including splenectomy.
Immunosuppression: Limited benefit may be observed using immunosuppression with cytotoxic agents. Azathioprine (150 mg/d) or cyclophosphamide (50-100 mg/d) has been used with some success. These cytotoxic drugs can cause myelosuppression, alopecia, hemorrhagic cystitis (cyclophosphamide), sterility, and secondary malignancy. They are given for a minimum duration and are withdrawn as soon as remission is achieved. Blood counts must be monitored during therapy.
Several studies reported improved platelet counts in patients with Helicobacter pylori –positive ITP following standard H pylori eradication therapy, with cohorts from Japan and Italy reporting higher response rates. However, a recent small, multicenter, randomized controlled study that evaluated 55 patients aged 4-18 years with chronic ITP found no beneficial effect of H pylori eradication on platelet recovery.
The anabolic steroid danazol (400-800 mg/d) has been shown to induce remission in certain patients. Cyclosporine and alfa-interferon have also been used. Plasmapheresis and extracorporeal protein A adsorption have been tried in desperate situations. The autoantibodies responsible for ITP are primarily IgG, and plasmapheresis is of limited value because more than half of the normal IgG pool is in the extravascular space.
Other evolving therapies for refractory ITP include autologous hematopoietic stem cell transplantation and anticytokine therapy with etanercept.
Urgent treatment for ITP patients with neurologic deficits or internal bleeding, or for emergency surgery, includes methylprednisolone, 30 mg/kg/day for 2 to 3 days, for a maximum of 1 g/day and/or IVIG, 1 g/kg/day for 2 to 3 days, combined with platelet transfusions. Vincristine, antifibrinolytic therapy e.g. tranexamic acid, recombinant factor VIIa, or continuous platelet transfusions should also be considered.
Relapsed ITP:
Treatment is indicated only for those with a platelet count of less than 30,000/mm3. Splenectomy (with a 66% response rate) is indicated in patients who relapse and do not respond to treatment with steroids, IVIG, or Rho(D) immune globulin. Rho(D) immune globulin is traditionally less effective in patients with ITP refractory to treatment.
Rituximab, a monoclonal antibody to CD20, has been used in patients with ITP with varying success. Rituximab has been reported to induce lasting remission in refractory ITP. A meta-analysis of adults suggests that splenectomy could be delayed and may be avoided altogether for patients who received rituximab earlier in the course of therapy. Disadvantages include cost, infusion reactions and lack on of long-term safety data.
Thrombotic Microangiopathies and Thrombotic Thrombocytopenic Purpura
Diagnosis
A pentad of signs is classically described—thrombocytopenia (platelet counts usually less than 20,000/mm3), microangiopathic hemolytic anemia, fever, renal dysfunction, and neurologic signs. A clinical triad of thrombocytopenia, red blood cell fragments (schistocytosis), and an increased lactate dehydrogenase (LDH) level is enough to suggest the diagnosis.
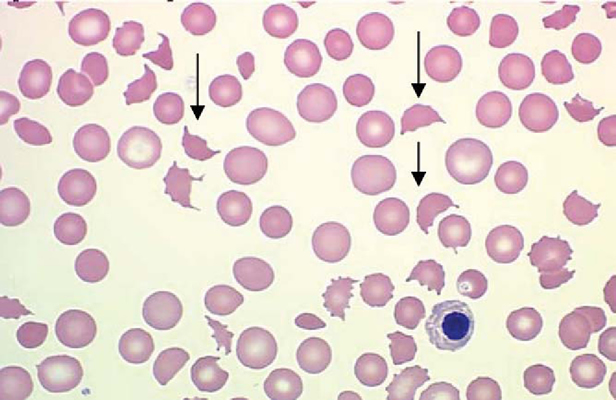
Peripheral blood smear of a patient with microangiopathic hemolytic anemia showing schistocytes (arrows).
Examination of the peripheral blood smear in patients with thrombocytopenia of unclear cause is imperative to exclude this diagnosis.
If severe renal failure is a prominent feature of the syndrome, the hemolytic-uremic syndrome may be a more likely diagnosis. Although ADAMTS13 (a zinc-containing metalloprotease enzyme that cleaves von Willebrand factor) levels can be measured, the diagnosis of TTP is a clinical one and results are often not available at the time of diagnosis.
Pathophysiology
Thrombotic microangiopathies are characterized by destructive thrombocytopenia, erythrocyte fragmentation, and tissue ischemia and necrosis, as evidenced by increased LDH levels. In nonacquired TTP, systemic clumping of platelets is caused by unusually large von Willebrand factor (vWF), often caused by a deficiency of the metalloproteinase ADAMTS13 that cleaves vWF into smaller multimers.
Causes of Thrombotic Thrombocytopenic Purpura
TTP can be familial or acquired. Familial TTP manifests in infancy or childhood, and often remits and relapses. Acquired TTP manifests in adults or older children and often occurs as a single acute episode.
Drug-induced TTP often occurs weeks after exposure. Medications commonly associated with this diagnosis include ticlopidine, mitomycin C, cyclosporine, tacrolimus, quinine and, less frequently, clopidogrel. Whole-body irradiation and organ transplantation also may result in a clinical syndrome similar to that of TTP.
Treatment
The treatment of childhood TTP (often related to ADAMTS13 deficiency) involves the transfusion of platelet-poor fresh-frozen plasma (FFP), FFP treated with organic solvent, or cryoprecipitate-poor plasma (cryosupernatant) every 3 weeks. The treatment of adults or older children with acquired TTP is by daily plasma exchange until platelet counts and LDH levels normalize. Patients not responding to these modalities might require the addition of steroids, consideration of splenectomy, or administration of vincristine. More recently, rituximab has been used in patients with refractory TTP, with varying efficacy. Platelets should not be transfused unless a life-threatening hemorrhage or intracranial bleed is present.
Post-Transfusion Purpura (PTP)
Diagnosis
PTP is a transfusion reaction characterized by severe thrombocytopenia lasting days to weeks after transfusion of platelet-containing products. Platelet antigen 1a, Pl(A1) antigen, is required to confirm the diagnosis.
Pathophysiology
Patients become sensitized to platelet antigen, most frequently platelet antigen 1a—Pl(A1) antigen—from prior transfusion of platelet-containing products or from pregnancy. This explains the much higher incidence among women. Pl(A1) antigen is also the platelet antigen most commonly involved in the pathophysiology of neonatal alloimmune purpura, thrombocytopenia that occurs in the neonatal period in the offspring of patients with PTP.
Treatment
The treatment of choice is IVIG, 400 mg/kg/day for 5 days or 1 g/kg/day for 2 days for severe thrombocytopenia. Further transfusions should be washed or Pl(A1) antigen–negative.
Heparin-Induced Thrombocytopenia (HIT)
Definition
HIT can be of two types:
Type I HIT occurs in about 10% of patients receiving heparin, usually within 2 days of heparin initiation, and platelet counts return to normal despite continued heparin exposure. As opposed to type II HIT, thrombocytopenia is mild (usually more than 100,000/mm3). It is nonimmune in origin and has no clinical consequences.
The incidence of type II HIT varies from 0.3% to 3% in patients who have received more than 4 days of heparin, most commonly unfractionated heparin, and has no relation to the heparin dose. It rarely occurs beyond 2 weeks of exposure.
The remainder of this discussion will focus on type II HIT, because type I HIT is of no clinical consequence.
Pathophysiology
The pathophysiology involves antibody formation against the heparin-platelet factor 4 complex, with resultant thrombosis. Thrombosis is usually venous, in the form of deep venous thrombosis or pulmonary embolism), but can be arterial as well, in the form of myocardial infarction or stroke.
Clinical Presentation
HIT is rare with platelet counts of less than 20,000/mm3; the average platelet count nadir is approximately 60,000/mm3. HIT has an earlier onset with re-exposure to heparin. A high index of suspicion is required and HIT should be considered in hospitalized patients with nosocomial thrombocytopenia. In addition, thrombosis is often associated with HIT and is usually asymptomatic.
Diagnosis
The diagnosis is clinical, despite the availability of adjunctive laboratory tests. These include the serotonin release assay, which is expensive and not widely available, but has high sensitivity and specificity and still remains the gold standard, the heparin-induced platelet aggregation test (HIPA), with a low sensitivity but high specificity, and the platelet-factor IV assays, highly sensitive but with a 10% to 20% clinical discordance with other tests. These adjunctive tests are often used in combination.
Prevention
Because they have been associated with lower rates of HIT (2.2% vs. 7.8% with unfractionated heparin), the use of low-molecular-weight heparin is believed to result in a decreased incidence of HIT. Prophylaxis with Fondaparinux (Arixtra) may also result in a lower incidence of HIT. Fondaparinux (Arixtra) is a synthetic sulfated pentasaccharide that inhibits factor Xa indirectly by binding to antithrombin III. Fondparinux does not bind to platelet factor 4, is structurally too short to induce an antibody response, and could in theory be a useful agent to treat HIT. The risk of HIT is thought to be negligible with Fondaparinux, as it has even been proposed as a useful alternate anticoagulant in HIT.
Treatment
Treatment involves discontinuation of all heparins, including IV line flushes and avoidance of warfarin until the platelet count normalizes. This approach carries a 30-day risk of thrombosis (de novo deep venous thrombosis) of 53%. Strategies to decrease the high risk of thrombosis include the following:
Anticoagulation with danaparoid, a heparinoid with a long half-life and 10% cross-reactivity with heparin, which requires monitoring of factor Xa activity.
Lepirudin, a renally cleared recombinant hirudin, can be used at a bolus dose of 0.1 to 0.4 μg/kg followed by a 0.1- to 0.15-mg/kg/hr infusion for a goal activated partial thromboplastin time (aPTT) of 1.5 to 2.5 times normal.
Argatroban, a direct thrombin inhibitor metabolized in the liver, can be used in patients with renal failure, 2 μg/kg/min, for a goal aPTT of 1.5 to 3 times normal. It has a short half-life—the anticoagulant effect wears off after 3 hours with normal hepatic function—and prolongs both the prothrombin time (PT) and the aPTT in a dose-dependent fashion. In patients with hepatic insufficiency, the dose should be decreased to 0.5 μg/kg/min.
Caution must be exercised with the use of argatroban, danaparoid, and lepirudin, because their effects cannot be reversed.
Case Report: A 69-year-old white female presented with a lower extremity extensive iliofemoral deep vein thrombosis after a right total knee arthroplasty and was subsequently found to have a pulmonary embolism. The patient was noted to have heparin flushes during her operation. Her platelet drop decreased >50% from baseline during initiation of antithrombotic therapy. She was started on subcutaneous fondaparinux 7.5 mg once daily injection. Her serotonin release assay and enzyme-linked immunosorbent assay for heparin antibodies were positive for HIT. Her platelet count nadir was 60 × 03/mm3 on day 5 and the platelet count rebounded after 8 days of fondaparinux therapy. No recurrent thrombotic or bleeding events were noted throughout her therapy. Anecdotal reports have shown that fondaparinux can be a useful agent to treat HIT with or without thrombosis. Clinical Thrombosis Center, Lovelace Medical Center, Albuquerque, NM, USA
Disseminated Intravascular Coagulation (DIC)
DIC is a systemic process that results in thrombosis and hemorrhage. Often, one presentation predominates and patients may have signs of bleeding or thrombosis. It is estimated to occur in 1% of hospitalized patients.
Pathophysiology
DIC represents a massive activation of the coagulation cascade that results in excessive production of thrombin, systemic intravascular fibrin deposition, and consumption of clotting factors and platelets. The initiating factor is the release of tissue factor caused by various mechanisms, including extensive endothelial injury and the monocyte response to endotoxin or various cytokines.
DIC can be acute, decompensated when the generation of clotting factor cannot keep up with the excessive consumption, or chronic, compensated when the clotting factors are generated at the same rate as they are consumed. Acute DIC occurs secondarily to various insults (Box 2) and its pathogenesis involves the massive generation of thrombin and consumption of coagulation factors. 
Clinical Features
Acute DIC manifests with bleeding and oozing from multiple sites, catheter access or mucosal surfaces, often in a critically ill patient with multisystem organ failure. Chronic DIC is most often associated with malignancy, usually solid tumors, and results from continuous slow exposure of blood to small amounts of tissue factor without overwhelming the compensatory mechanisms that regenerate depleted factors. It most often manifests clinically with thrombosis rather than hemorrhage.
Diagnosis
The diagnosis of acute DIC relies on the following: history and clinical setting, with particular attention to trauma, sepsis, malignancy, pregnancy, and miscarriages; moderate to severe thrombocytopenia; evidence of microangiopathic hemolysis on the peripheral smear (e.g., the presence of schistocytes); and suggestive laboratory test results. Clinically significant DIC is unlikely in the presence of normal d-dimers. Prolonged PT and aPTT can also be noted, as well as decreased fibrinogen levels; fibrinogen, however, is an acute-phase reactant and may be falsely normal. The thrombin time (TT) is prolonged, whereas antithrombin III (ATIII), protein C, and protein S levels are often depressed.
Chronic DIC may manifest with more subtle laboratory results—smear microangiopathy and an elevated d-dimer level may be the only laboratory finding.
Treatment
Acute DIC in the setting of sepsis, trauma, or burns carries 40% to 80% mortality. Increasing age and severity of multiorgan failure represent worse prognostic factors.
Treatment is largely supportive, with platelet or FFP transfusions, or both, in bleeding patients and in those at high risk for bleeding. Cryoprecipitate transfusion in patients with a fibrinogen level lower than 1.0 gm/L is often considered although its benefit is difficult to demonstrate.
Heparin was not shown to be beneficial in acute DIC in controlled trials, and its role is limited to the treatment of DIC associated with the retained products of gestation or with giant hemangiomas. Heparin has been used with varying efficacy in patients with DIC and disseminated malignancy.
Emerging but not yet validated treatment for DIC includes protein C concentrates for patients with homozygous protein C deficiency, antithrombin, and activated protein C, which has demonstrated survival benefit in severe sepsis.
References:
Kurata Y, Hayashi S , Kiyoi T. Diagnostic value of tests for reticulated platelets, plasma glycocalicin, and thrombopoietin levels for discriminating between hyperdestructive and hypoplastic thrombocytopenia. Am J Clin Pathol. 115: 2001; 656-664.
Kuter DJ, Begley CG. Recombinant human thrombopoietin: Basic biology and evaluation of clinical studies. Blood. 100: 2002; 3457-3469.
Mozes MF, Spigos DG , Pollak R. Partial splenic embolization, an alternative to splenectomy—results of a prospective, randomized study. Surgery. 96: 1984; 694-702.
Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 346: 2002; 995-1008.
George JN, Woolf SH , Raskob GE. Idiopathic thrombocytopenic purpura: A practice guideline developed by explicit methods for the American Society of Hematology. Blood. 88: 1996; 3-40.
Nilsson T, Norberg B. Thrombocytopenia and pseudothrombocytopenia: a clinical and laboratory problem. Scand J Haematol. 1986 Oct. 37(4):341-6.
Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009 Feb 21. 373(9664):641-8.
Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008 Feb 2. 371(9610):395-403.
Auger S, Duny Y, Rossi JF, Quittet P. Rituximab before splenectomy in adults with primary idiopathic thrombocytopenic purpura: a meta-analysis. Br J Haematol. 2012 Aug. 158(3):386-98.
Jackson S, Beck PL, Pineo GF, Poon MC. Helicobacter pylori eradication: novel therapy for immune thrombocytopenic purpura? A review of the literature. Am J Hematol. 2005 Feb. 78(2):142-50.
Moake JL. Thrombotic microangiopathies. N Engl J Med. 347: 2002; 589-600.
McCrae KR, Herman JH. Posttransfusion purpura: Two unusual cases and a literature review. Am J Hematol. 52: 1996; 205-211.
Warkentin TE, Chong BH , Greinacher A. Heparin-induced thrombocytopenia: towards consensus. Thromb Haemost. 79: 1998; 1-7.
Kuo KH, Kovacs MJ. Fondaparinux: a potential new therapy for HIT. Hematology. 2005;10(4):271–275.
Warkentin TE. Current agents for the treatment of patients with heparin-induced thrombocytopenia. Curr Opin Pulm Med. 8: 2002; 405-412.
Alex C Spyropoulos The use of fondaparinux for the treatment of venous thromboembolism in a patient with heparin-induced thombocytopenia and thrombosis caused by heparin flushes https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2500261/
Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: The role of heparin therapy. Blood. 60: 1982; 284-287.
Keywords:
thrombocytopenia definition, thrombocytopenia causes, thrombocytopenia symptoms, thrombocytopenia purpura, thrombocytopenia treatment, thrombocytopenia in pregnancy, thrombocytopenia workup, itp icd 10 code, thrombocytopenia icd 10 dx code, thrombocytopenia definition medical, thrombocytopenia causes in adults, thrombocytopenia causes in child, thrombocytopenia causes medication, thrombocytopenia symptoms and signs, thrombocytopenia symptoms in adults, thrombocytopenia symptoms and treatment. thrombocytopenic purpura symptoms, thrombocytopenic purpura treatment, thrombocytopenic purpura ttp, idiopathic thrombocytopenic purpura (itp).




I have platelets above 50 thousand and 90 thousand only
Hello,
Thank you for your message.
Can you give me some more information regarding the duration of your low platelet count, your past medical history, your current medications and alcohol intake along with your most recent full blood picture to have a look and advice?
Regards,