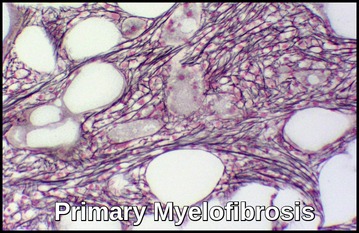
Primary Myelofibrosis
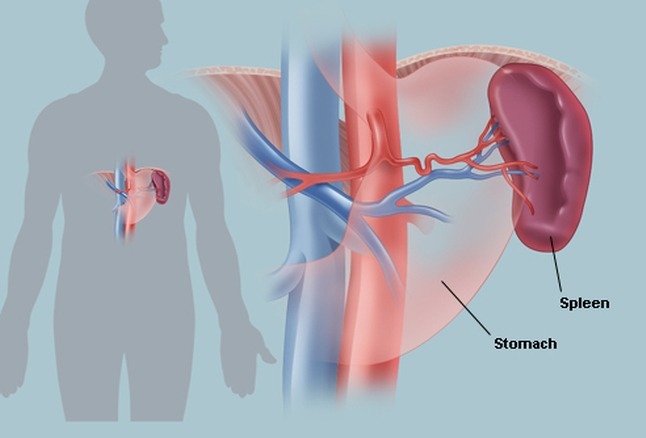
Primary myelofibrosis (PMF) is a chronic myeloproliferative neoplasm characterized by progressive bone marrow fibrosis, extramedullary hematopoiesis, massive splenomegaly, and anemia often accompanied by nucleated red cells and teardrop-shaped erythrocytes. As the spleen and liver enlarge due to compensatory hematopoiesis, patients may develop abdominal discomfort, infarction, portal hypertension, hypersplenism, plasma volume expansion, and an increased risk of splanchnic vein thrombosis. These features reflect the underlying marrow failure and redistribution of blood cell production, which are central to the disease biology of PMF.

Axial portal venous phase CT showing massive splenomegaly in primary myelofibrosis, with multiple wedge-shaped and linear low-attenuation areas consistent with splenic infarctions (white arrows).

Peripheral smear demonstrating teardrop cells (dacrocytes), a hallmark feature of primary myelofibrosis.
Myelofibrosis may be primary or secondary to several hematologic, malignant, and non-malignant conditions e.g. Polycythemia Vera (PRV), Essential Thrombocythemia (ET), Hairy Cell Leukemia, cancer with bone marrow metastases, osteomyelitis and TB.
Primary Myelofibrosis (PMF) is more common than secondary myelofibrosis and results from a neoplastic transformation of a multipotent bone marrow stem cell. These PMF progeny cells stimulate bone marrow fibroblasts to secrete excessive collagen.
The disease is usually idiopathic.
In this disorder, marrow fibrosis and extramedullary hematopoiesis (primarily in the liver and spleen) predominate. The fibrosis is probably reactive.
Affected individuals may not have symptoms at the time of diagnosis (asymptomatic) and may remain symptom-free for many years. Eventually, affected individuals may develop fatigue, fever, frequent infections, pale skin, night sweats and unexplained weight loss.
There is anemia with anisocytosis, poikilocytosis, and ‘teardrop’ red blood cells (dacrocytes).
The leukoerythroblastic blood picture is, as the name implies, characterized by the presence of immature forms of red and white cells in the peripheral blood. Normoblasts as well as myeloblasts (even in the absence of acute leukemia), promyelocytes, myelocytes and metamyelocytes may be present.

Leukoerythroblastic blood film demonstrating nucleated RBCs and teardrop cells (dacrocytes) in primary myelofibrosis.
Serum LDH level is often elevated.
Platelet counts initially may be high, normal, or decreased; however, thrombocytopenia tends to supervene as the disorder progresses.
Bone marrow failure eventually occurs, with consequent anemia and thrombocytopenia.
Rapidly progressive, chemotherapy-incurable acute leukemia (usually M7 AML) develops in about 10% of patients.
Diagnosis:
- FBC and blood smear.
- Bone marrow examination. Bone marrow aspiration is usually dry. Because the demonstration of bone marrow fibrosis is required and fibrosis may not be uniformly distributed, a biopsy should be repeated at a different site if the first biopsy is nondiagnostic.
- Cytogenetic studies of bone marrow help exclude chronic myelogenous leukemia (CML), myelodysplastic syndrome, or other chronic myeloid disorders. However, as mentioned, these studies may be difficult to obtain due to the “dry tap” on bone marrow aspirates in over 50% of patients with primary myelofibrosis. Fluorescent in situ hybridization (FISH) studies or polymerase chain reaction (PCR) assay testing for bcr:abl may help exclude CML (this may also be performed on peripheral blood). FISH studies for abnormalities associated with myelodysplastic syndromes, such as del 7, 7q-, and 5q-, may also be helpful.
- About 50% of patients have a JAK2 mutation. Calreticulin (CALR) mutations were recently described in JAK2 and thrombopoietin receptor gene (myeloproliferative leukemia, MPL) unmutated primary myelofibrosis and essential thrombocythemia. Among 254 study patients, 147 (58%) harbored JAK2, 63 (25%) CALR and 21 (8.3%) MPL mutations; 22 (8.7%) patients were negative for all three mutations (triple-negative myelofibrosis), whereas one patient expressed both JAK2 and CALR mutations.
- U&Es.
- LFTs.
- Serum LDH and uric acid.
- Abdominal USS.
- CT scan of the abdomen.
- FDG PET/CT scan.

FDG PET/CT MIP image in primary myelofibrosis showing diffuse FDG uptake throughout the bones, with prominent hepatosplenomegaly reflecting active extramedullary hematopoiesis.
Treatment:
Historically, therapy for primary myelofibrosis was mainly supportive. Patients received transfusions as needed.
Allopurinol for gout prevention.
Thrombocytosis could be managed with Hydroxyurea and other palliative agents.
Low-risk, asymptomatic patients may be observed without intervention.
Patients with milder disease may still be treated with supportive therapies.
Ruxolitinib (Jakavi/Jakafi) is a JAK1/JAK2 inhibitor and the first FDA-approved therapy for primary myelofibrosis, offering significant reductions in splenomegaly and symptom burden. The recommended starting dose is 15–20 mg twice daily, depending on baseline platelet count (20 mg BID for platelets >200 ×10⁹/L and 15 mg BID for 100–200 ×10⁹/L). Dose adjustments are individualized based on response and cytopenias. Ruxolitinib remains the cornerstone of myelofibrosis management, improving quality of life and constitutional symptoms while slowing disease-related inflammation and cytokine-mediated complications.
Momelotinib (Ojjaara) Momelotinib (Ojjaara) is a JAK1/JAK2 and ACVR1 inhibitor approved for the treatment of primary myelofibrosis, especially in patients with baseline anemia or transfusion dependence. The recommended dose is 200 mg orally once daily with food. Unlike ruxolitinib and other JAK inhibitors, Momelotinib can improve anemia by reducing hepcidin levels and enhancing iron mobilization, while still providing effective control of splenomegaly and constitutional symptoms. Clinical trials have shown reductions in spleen volume, improved symptom burden, and higher rates of transfusion independence, making Momelotinib a valuable option for patients who are anemic at diagnosis or intolerant of alternative JAK inhibitors.

Momelotinib (Ojjaara) tablets in 100 mg, 150 mg, and 200 mg strengths—anemia-targeted JAK inhibitor therapy for primary myelofibrosis.
Androgens, splenectomy, chemotherapy, splenic embolization and radiation therapy have been used for palliation.
Temporary use of low-dose corticosteroids may relieve symptoms.
For younger patients with advanced disease, allogeneic stem cell transplantation may be beneficial. Nonmyeloablative allogeneic stem cell transplantation has been successfully used even in older patients.
Although thalidomide and lenalidomide can alleviate anemia in myelofibrosis, their use is limited by their respective potential to cause peripheral neuropathy and myelosuppression. Pomalidomide is a second-generation thalidomide analogue with reduced toxicity and enhanced anticancer and immunological activity.
- The median survival is 5 yr from the onset, but variation is wide; some patients have a rapidly progressing disorder with short survival and some have a delay in initial diagnosis.
- Unfavorable prognostic markers include Hb < 10 g/dL, history of transfusions, leukocytosis and leukopenia, and platelet count < 100,000/μL.
- Patients in the least favorable risk group usually survive < 1 yr.
- No treatment reverses or controls the underlying process except for allogeneic stem cell transplant.
Summary:
Primary myelofibrosis (PMF) is a rare chronic myeloproliferative neoplasm characterized by abnormal hematopoiesis and progressive bone marrow fibrosis. The disease arises from a mutation in a single hematopoietic stem cell—most commonly involving JAK2, CALR, or MPL—leading to clonal expansion and the gradual replacement of normal marrow with fibrous tissue. As fibrosis and abnormal cell proliferation increase, the production of red cells, white cells, and platelets becomes impaired, forcing hematopoiesis to shift to extramedullary sites such as the spleen and liver.
Clinical presentation varies widely. Many patients are asymptomatic at diagnosis, while others experience fatigue, anemia, early satiety, fever, night sweats, weight loss, or recurrent infections. Massive splenomegaly is a hallmark of PMF, and hepatomegaly may also develop as the disease progresses. Over time, cytopenias, leukoerythroblastic changes, and systemic symptoms reflect worsening marrow failure. Approximately half of patients carry a JAK2 V617F mutation, although its precise role in disease pathogenesis continues to be explored.
Questions and Answers:
What is primary myelofibrosis and how does it affect the bone marrow?
Primary myelofibrosis is a chronic myeloproliferative neoplasm in which a mutated stem cell leads to abnormal marrow hematopoiesis and progressive fibrosis. As scarring replaces normal marrow, the production of red cells, white cells, and platelets becomes severely impaired.
What causes teardrop cells (dacrocytes) in primary myelofibrosis?
Teardrop cells form when erythrocytes are forced out of a fibrotic marrow, producing their characteristic distorted, tear-shaped appearance. They are a classic peripheral smear feature of marrow fibrosis in PMF.
What does a leukoerythroblastic blood film indicate in primary myelofibrosis?
A leukoerythroblastic film—showing nucleated red cells and early myeloid precursors—reflects severe bone marrow disruption with extramedullary hematopoiesis, commonly seen in advanced PMF.
Why does primary myelofibrosis cause massive splenomegaly?
As the bone marrow becomes fibrotic and fails to produce blood cells effectively, hematopoiesis shifts to the spleen, causing progressive enlargement that may lead to abdominal discomfort, infarctions, and portal hypertension.
How is primary myelofibrosis diagnosed on imaging?
CT and ultrasound typically demonstrate massive splenomegaly, while PET/CT may show diffuse bone uptake and hepatosplenomegaly associated with extramedullary hematopoiesis. Imaging supports diagnosis and staging.
What does PET/CT show in patients with primary myelofibrosis?
FDG PET/CT often demonstrates diffuse osseous uptake due to marrow hyperactivity or inflammation, along with marked hepatosplenomegaly, reflecting the shift of blood cell production outside the marrow.
Which mutations are associated with primary myelofibrosis?
The majority of cases carry mutations in JAK2, CALR, or MPL. JAK2 V617F occurs in about half of patients, while CALR and MPL mutations account for most of the remaining cases.
What are the first-line treatments for primary myelofibrosis?
Ruxolitinib is the first-line JAK inhibitor for controlling splenomegaly and symptoms. Dose is based on platelet count, typically 15–20 mg twice daily. Allogeneic stem cell transplantation remains the only curative option.
What alternatives exist for patients who cannot tolerate ruxolitinib?
Approved alternatives include Momelotinib (beneficial in anemic patients), Fedratinib (effective post-ruxolitinib), and Pacritinib (preferred when platelets <50 × 10⁹/L). These options address key unmet needs such as anemia and thrombocytopenia.
How does Momelotinib help anemic patients with primary myelofibrosis?
Momelotinib inhibits JAK1/JAK2 and ACVR1, lowering hepcidin levels and improving iron availability. At 200 mg once daily, it reduces spleen size and improves anemia, helping patients achieve transfusion independence.
Can primary myelofibrosis be cured?
The only potentially curative therapy is allogeneic stem cell transplantation, reserved for selected patients with high-risk disease or adverse mutations. All other therapies focus on symptom control and improving quality of life.
What symptoms suggest progression of primary myelofibrosis?
Worsening anemia, increased spleen size, night sweats, weight loss, bone pain, recurrent infections, and rising transfusion needs often indicate disease progression and may require treatment escalation.
What is the role of erythropoietin (EPO) in primary myelofibrosis?
In primary myelofibrosis, erythropoietin (EPO) levels are often elevated as the body attempts to compensate for severe anemia caused by marrow fibrosis. However, because the fibrotic marrow cannot effectively produce red cells, this increased EPO response is inadequate, and many patients do not achieve meaningful improvement in hemoglobin. EPO-stimulating agents may help a small subset of patients with low endogenous EPO levels, but their overall effectiveness in PMF is limited.
References:
Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT). Leuk Res. 2007;31(6):737–740.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390.
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807.
Gangat N, Caramazza D, Vaidya R, et al. DIPSS-plus: A refined Dynamic International Prognostic Scoring System for primary myelofibrosis incorporating karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–397.
Oon SF, Singh D, Tan TH, et al. Primary myelofibrosis: Spectrum of imaging features and disease-related complications. Insights Imaging. 2019;10:71. https://doi.org/10.1186/s13244-019-0758-y
National Organization for Rare Disorders (NORD). Primary Myelofibrosis. Available at: https://rarediseases.org/rare-diseases/primary-myelofibrosis/
Keywords:
primary myelofibrosis, PMF, myeloproliferative neoplasm, bone marrow fibrosis, leukoerythroblastic blood film, teardrop cells, dacrocytes, splenomegaly, hepatosplenomegaly, extramedullary hematopoiesis, JAK2 mutation, CALR mutation, MPL mutation, JAK inhibitors, ruxolitinib, fedratinib, momelotinib, pacritinib, DIPSS plus, PET CT myelofibrosis, CT splenomegaly, marrow failure, myelofibrosis treatment, Jakavi, Jakafi, Ojjaara, myelofibrosis prognosis, myelofibrosis symptoms, myelofibrosis diagnosis, marrow scarring, myelofibrosis imaging, MF anemia, PMF management, MF splenic infarction, bone marrow biopsy fibrosis, MF blood film findings, megakaryocyte atypia, myelofibrosis complications


Request Online Consultation With Dr M Abdou
Fee: US$100
Secure payment via PayPal (credit and debit cards accepted)
Pay Now



I have a patient of primary myelofibrosis intermediate risk with hepatosplenomegaly on ruxolitinib 25 mg bd who presented with high counts of 67 thrombocytopenia tophaceoys gout and mild pulmonary congestion likely secondary to mild LVF and AKI 1 with a edgr of 33
I reduced his ruxolitinib at 5mg bd and sent blood for flow as blood film showed blasts at 1.6%
Was the the right reduction of ruxolitinib
We are investigating the cause of HF if he has transformed to aml would you give hydroxy?
Hi,
Thank you for your message.
You have to follow the Ruxolitinib dosing guidelines. Doses may be titrated based on safety and efficacy.
If efficacy is considered insufficient and platelet and neutrophil counts are adequate, doses may be increased by a maximum of 5 mg twice daily. The starting dose should not be increased within the first four weeks of treatment and thereafter no more frequently than at 2-week intervals. For platelets 50 to less than 100 x 10(9)/L: 5 mg orally twice a day should be okay.
Duration of therapy: 6 months if no spleen reduction or symptom improvement.
BW,