Checkpoint Inhibitors

Checkpoint inhibitors restore T-cell activation and improve immune recognition of tumor cells, a key mechanism in modern cancer immunotherapy.
A key function of the immune system is its ability to distinguish healthy self-cells from harmful “foreign” cells, ensuring precise immune surveillance and protection. Cancer cells can evade this process by exploiting immune-checkpoint pathways, allowing tumors to grow unchecked. Checkpoint inhibitors work by blocking these pathways and restoring the immune system’s ability to recognize and attack cancer.
Immune system uses “checkpoints” – molecules on certain immune cells that need to be activated (or inactivated) to start an immune response.
Cancer cells sometimes find ways to use these checkpoints to avoid being attacked by the immune system. They can hide by fooling immune cells into thinking they are not foreign at all. To determine friend or foe, immune cells check for a protein. If they find it on cancer cells, they appear as friends.
Drugs that target these checkpoints “checkpoints inhibitors” hold a lot of promise as cancer treatments. They do just what the name suggests –they block the checkpoint that looks for friend or foe. Seeing no friend because they are blocked, immune cells mount a defense.
Immune checkpoint inhibitors are monoclonal antibodies that block proteins, called “checkpoints,” expressed by tumor cells to inhibit the immune response. The development of this therapy for hematologic cancers has lagged behind that of solid tumors, but new data at the 2015 ASH annual meeting suggest the promise may be similar.
In solid tumors, several checkpoint inhibitors have now been granted regulatory approval for single-agent use in advanced disease. The most recent approvals, granted to both nivolumab and pembrolizumab, were for single-agent use in advanced non-small cell lung cancer. As yet, no checkpoint inhibitor has been granted an indication for a hematologic cancer or lymphoproliferative disorder, but it appears that at least some of the approvals, when they are granted, will be for a checkpoint inhibitor in combination with a second agent.
Checkpoint inhibitors scored some impressive successes in recent years, particularly in some patients with metastatic melanoma, metastatic non-small cell lung cancer (NSCLC), advanced renal cell carcinoma or classical Hodgkin lymphoma, and are showing promise in clinical trials involving patients with other types of cancer.

PD-1 and PD-L1 checkpoint inhibitors block the PD-1/PD-L1 interaction, allowing T cells to recognize and attack cancer cells more effectively.
Drugs that target The programmed cell death-1 (PD-1) receptor or one of its ligands, (PD-L1):
PD-1 is a checkpoint protein on immune T cells. It normally acts as a type of “off switch” that helps keep the T cells from attacking other cells in the body. It does this when it attaches to PD-L1, a protein on some normal (and cancer) cells. When PD-1 binds to PD-L1, it basically tells the T cell to leave the other cell alone. Some cancer cells have large amounts of PD-L1, which helps them evade immune attack.
Monoclonal antibodies that target either PD-1 or PD-L1 can boost the immune response against cancer cells and have shown a great deal of promise in treating certain cancers.
PD-1 inhibitors: Examples of drugs that target PD-1 include:
Keytruda (pembrolizumab) is a PD-1 checkpoint inhibitor widely used to boost antitumor immunity across multiple cancer types.

Opdivo (nivolumab) is a PD-1 checkpoint inhibitor that restores antitumor immunity and is widely used across multiple solid tumors.
These drugs have been shown to be helpful in treating several types of cancer, including metastatic melanoma, non-small cell lung cancer, advanced renal cell carcinoma, head and neck cancers, and Hodgkin lymphoma. They are also being studied for use against many other types of cancer.
PD-L1 inhibitors: An example of a drug that targets PD-L1 is:
Tecentriq (atezolizumab) is a PD-L1 checkpoint inhibitor that boosts immune recognition of cancer cells and improves antitumor activity across several malignancies.
Atezolizumab (Tecentriq)
This drug can be used to treat bladder cancer, and is also being studied for use against other types of cancer.
One concern with all of these drugs is that they can allow the immune system to attack some normal organs in the body, which can lead to serious side effects in some people.
Common immune-related adverse events of these drugs can include fatigue, cough, nausea, loss of appetite, skin rash, and itching. Less often they can cause more serious problems in the lungs, intestines, liver, kidneys, endocrine glands, or other organs.
Many other drugs that target either PD-1 or PD-L1 are now being tested in clinical trials as well, both alone and combined with other drugs.
Drugs that target Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4):
CTLA-4 is another protein on some T cells that acts as a type of “off switch” to keep the immune system in check.

Yervoy (ipilimumab) is a CTLA-4 checkpoint inhibitor that strengthens T-cell activation and improves immune control of cancer across selected indications.
CTLA-4 inhibitors:
Ipilimumab (Yervoy) is a monoclonal antibody that attaches to CTLA-4 and stops it from working. This can boost the body’s immune response against cancer cells.
This drug is used to treat metastatic melanoma. It is also being studied for use against other cancers.
Because ipilimumab affects the immune system, it can sometimes cause serious or even life-threatening side effects. In fact, compared to drugs that target PD-1 or PD-L1, serious side effects seem to be more likely with ipilimumab.
Conclusion:
Immune checkpoint inhibitors targeting CTLA-4 and PD-1/PD-L1 have transformed cancer therapy, producing durable antitumor responses in melanoma, squamous NSCLC, and an expanding range of malignancies. Their use, however, is associated with a distinct spectrum of immune-related adverse events (irAEs) that differ fundamentally from toxicities seen with conventional chemotherapy. Early recognition and timely management of irAEs—most often with temporary immunosuppression—are essential to prevent severe or life-threatening complications. Established clinical algorithms now guide the management of dermatologic, gastrointestinal, hepatic, pulmonary, and endocrine toxicities seen with agents such as ipilimumab, nivolumab, and pembrolizumab. While most irAEs are reversible when treated promptly with corticosteroids or second-line immunosuppressants such as TNF-α inhibitors or mycophenolate mofetil, delayed intervention can result in significant morbidity or mortality. As checkpoint inhibitor use continues to expand, heightened clinician awareness and adherence to evidence-based toxicity management remain critical to optimizing patient outcomes.
Questions and Answers:
What are checkpoint inhibitors and how do they work?
Checkpoint inhibitors are immunotherapy drugs that block inhibitory receptors such as PD-1, PD-L1, and CTLA-4 on T cells. By releasing these “immune brakes,” they restore T-cell activation and enhance the body’s ability to recognize and destroy cancer cells.
Which cancers are treated with checkpoint inhibitors?
Checkpoint inhibitors are approved for multiple cancers including melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, urothelial carcinoma, head and neck cancers, triple-negative breast cancer, colorectal cancers with MSI-H or dMMR, and others. Indications continue to expand based on emerging clinical evidence.
What are the most common immune-related adverse events (irAEs)?
The most frequent irAEs include skin rash, pruritus, diarrhea or colitis, hepatitis, pneumonitis, and endocrine abnormalities such as hypothyroidism, hyperthyroidism, and hypophysitis. Severity ranges from mild to life-threatening, making early recognition essential.
How are immune-related adverse events managed?
Most irAEs respond well to early immunosuppression, typically with systemic corticosteroids. Severe or steroid-refractory cases may require targeted immunosuppressants such as infliximab, mycophenolate mofetil, or other agents depending on organ involvement. Treatment guidelines from ASCO and ESMO provide structured algorithms.
Are checkpoint inhibitor responses durable?
Yes. A unique advantage of checkpoint inhibitors is the potential for durable long-term remissions, even after treatment discontinuation. This is particularly well documented in melanoma, NSCLC, and renal cell carcinoma, with a subset of patients achieving multi-year survival gains.
What is the difference between PD-1, PD-L1, and CTLA-4 inhibitors?
PD-1 inhibitors (nivolumab, pembrolizumab) block the PD-1 receptor on T cells.
PD-L1 inhibitors (atezolizumab, durvalumab, avelumab) block PD-L1 on tumor cells.
CTLA-4 inhibitors (ipilimumab) act earlier in the immune activation pathway.
These mechanisms influence toxicity patterns, combination strategies, and clinical indications.
Can checkpoint inhibitors be combined with other treatments?
Yes. Combination therapy—such as nivolumab plus ipilimumab, or checkpoint inhibitors paired with chemotherapy, targeted therapy, or radiotherapy—can improve efficacy but increases toxicity risks. Combination decisions depend on tumor type, stage, biomarkers, and patient fitness.
What biomarkers predict response to checkpoint inhibitors?
Key biomarkers include PD-L1 expression, tumor mutational burden (TMB), microsatellite instability (MSI-H), mismatch repair deficiency (dMMR), and specific gene signatures. These biomarkers help guide treatment selection and predict likelihood of benefit.
Are checkpoint inhibitors effective in hematologic malignancies?
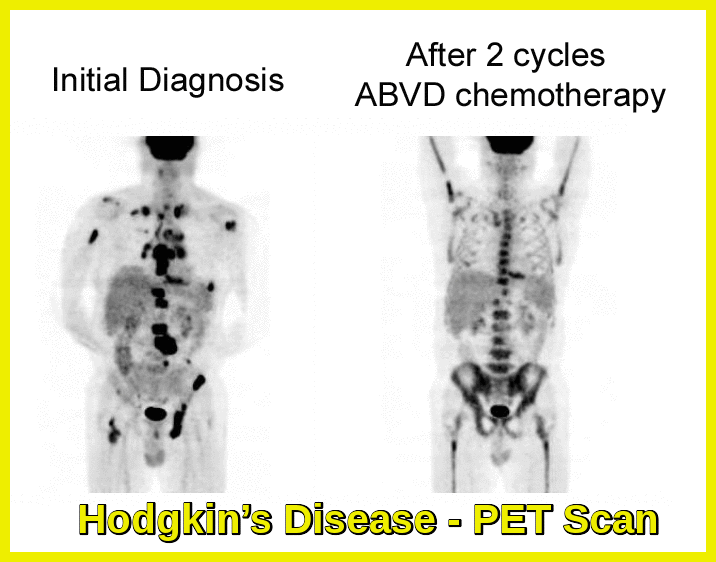
Yes, particularly in classical Hodgkin lymphoma where PD-1 blockade produces high response rates. Research continues in non-Hodgkin lymphomas, multiple myeloma, and myeloid neoplasms, though responses vary by disease subtype.
Can patients remain on immunotherapy long term?
Most patients receive checkpoint inhibitors for a defined period (1–2 years in many protocols). Long-term therapy may continue if benefit persists and toxicity remains manageable, though some patients maintain durable remission after stopping treatment.
References:
American Cancer Society. Immune Checkpoint Inhibitors to Treat Cancer. Cancer.org.
https://www.cancer.org/cancer/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html
Accessed August 2016.
Bosworth T. Checkpoint Inhibitors in Hematologic Cancers: Overview. Medscape.
https://www.medscape.com/viewarticle/857469
Accessed January 2016.
Postow MA. Managing Immune Checkpoint–Blocking Antibody Side Effects. Am Soc Clin Oncol Educ Book. 2015;35:76–83.
doi:10.14694/EdBook_AM.2015.35.76. PMID: 25993145.
Villadolid J, Amin A. Immune Checkpoint Inhibitors in Clinical Practice: Update on Immune-Related Toxicities.
https://pubmed.ncbi.nlm.nih.gov/26629425/
Accessed October 2015.
Dana-Farber Cancer Institute. What Is a Checkpoint Inhibitor?
https://blog.dana-farber.org/insight/2015/09/what-is-a-checkpoint-inhibitor/
Accessed July 2016.
Charbonneau D. Your Microbiome and Cancer: Interaction With Checkpoint Inhibitors.
https://eyeviewkamloops.wordpress.com/tag/checkpoint-inhibitors/
Accessed June 2016.
Haslam A., Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy. JAMA Netw Open. 2019;2(5):e192535.
doi:10.1001/jamanetworkopen.2019.2535.
Ribas A., Wolchok JD. Cancer Immunotherapy Using Checkpoint Blockade. N Engl J Med. 2018;379:2185–2195.
doi:10.1056/NEJMra1706162. (Cornerstone NEJM review; still high-impact)
Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer. 2012;12:252–264.
doi:10.1038/nrc3239. (Classic Nature Reviews reference with enduring relevance)
Postow MA., Sidlow R., Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med. 2018;378:158–168.
doi:10.1056/NEJMra1703481.
ESMO Guidelines Committee; Haanen JBAG et al. Management of Toxicities From Immunotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2022;33(12):1217–1238.
doi:10.1016/j.annonc.2022.08.003.
ASCO Expert Panel; Brahmer JR et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy. J Clin Oncol. 2021;39(36):4073–4126. doi:10.1200/JCO.2021.39.36.4073.
Keywords:
checkpoint inhibitors, immune checkpoint inhibitors, PD-1 inhibitors, PD-L1 inhibitors, CTLA-4 inhibitors, immunotherapy for cancer, cancer immunotherapy, how checkpoint inhibitors work, PD-1 PD-L1 pathway, CTLA-4 pathway, T-cell activation cancer, immunotherapy mechanism of action, nivolumab Opdivo, pembrolizumab Keytruda, atezolizumab Tecentriq, durvalumab Imfinzi, avelumab Bavencio, ipilimumab Yervoy, PD-1 vs PD-L1 inhibitors, checkpoint inhibitor drugs list, checkpoint inhibitor side effects, immune-related adverse events, irAEs management, immunotherapy toxicity management, steroid treatment for irAEs, immunotherapy colitis, immunotherapy hepatitis, immunotherapy pneumonitis, endocrine irAEs, hypothyroidism immunotherapy, hypophysitis immunotherapy, MSI-H immunotherapy, dMMR immunotherapy, tumor mutational burden immunotherapy, TMB high cancers, biomarkers for checkpoint inhibitors, immunotherapy approved cancers, melanoma immunotherapy, NSCLC immunotherapy, renal cell carcinoma immunotherapy, Hodgkin lymphoma PD-1 therapy, triple negative breast cancer immunotherapy, immunotherapy combination treatments, nivolumab pembrolizumab indications, ipilimumab toxicity, PD-1 PD-L1 cancer treatment, durable responses immunotherapy, immunotherapy for solid tumors, immunotherapy for hematologic cancers, checkpoint inhibitors in lymphoma, immune checkpoint blockade, ASCO immunotherapy guidelines, ESMO immunotherapy guidelines, cancer immune evasion, T-cell immune response, oncology immunotherapy education, Ask Hematologist, Dr Moustafa Abdou.


Request Online Consultation With Dr M Abdou
Fee: US$100
Secure payment via PayPal (credit and debit cards accepted)
Pay Now



After I read the article, especially when I am interested in the field of immunity and how to strengthen immunity in the body, what are the latest statistics for this treatment? And what are the countries that have used such drugs
Hi Mohamed,
Thank you for your comment.
Checkpoint inhibitors are in use in the UK for several years now. In Hematology we use them in relapsed or refractory classical Hodgkin lymphoma e.g. nivolumab or pembrolizumab.
BW,