Myelodysplastic Syndrome
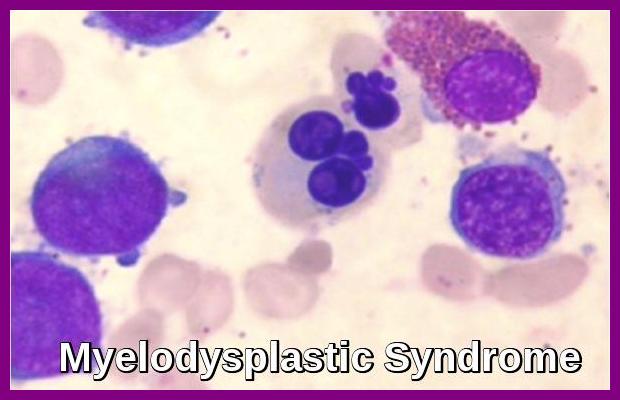
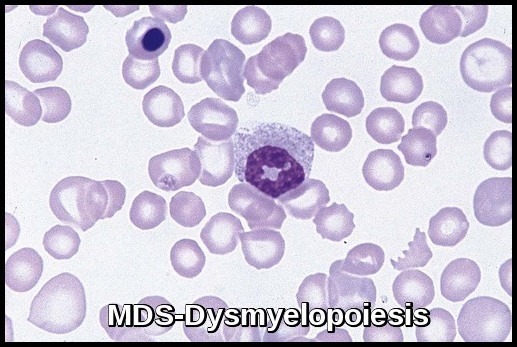
Bone marrow aspirate demonstrating dysplastic erythroid and myeloid precursors with abnormal nuclear segmentation, characteristic of Myelodysplastic Syndrome (MDS).
Myelodysplastic Syndrome (MDS) is a clonal bone marrow failure disorder characterized by ineffective hematopoiesis, persistent cytopenias, and a variable risk of progression to acute myeloid leukemia (AML). This group of hematologic neoplasms arises when hematopoietic stem cells acquire genetic or epigenetic mutations that impair normal blood cell maturation across one or more lineages—erythroid, granulocytic, or megakaryocytic. The resulting dysplasia leads to abnormal cell morphology, poor marrow output, and peripheral cytopenias such as anemia, neutropenia, and thrombocytopenia. Because of its potential to transform into AML, MDS is often regarded as a preleukemic condition requiring careful diagnostic evaluation and risk-stratified management.
Myelodysplastic syndromes are classified as clonal stem cell disorders that cause ineffective blood cell production and varying degrees of cytopenia. According to World Health Organization (WHO) classification and the International Consensus Classification (ICC), MDS encompasses a spectrum ranging from low-risk disease with isolated cytopenia to high-risk subtypes with increased blasts and complex cytogenetic abnormalities. Modern molecular profiling has identified recurrent mutations in genes such as SF3B1, TET2, ASXL1, TP53, and RUNX1, which have prognostic and therapeutic significance.
MDS primarily affects older adults (over 65 years), although younger individuals can also develop the condition. Early stages are driven by increased apoptosis (programmed cell death), while advanced stages show accumulating mutations and clonal evolution, leading to expansion of leukemic blasts and potential progression to AML. Although the bone marrow is typically hypercellular, a hypocellular variant (hypoplastic MDS) may mimic aplastic anemia. Accurate diagnosis requires bone marrow biopsy, cytogenetic and molecular testing, and risk assessment using the Revised International Prognostic Scoring System (IPSS-R) or the newer IPSS-M.
Early diagnosis and individualised, risk-adapted therapy—including hypomethylating agents, lenalidomide, luspatercept, and allogeneic stem cell transplantation—can improve outcomes, reduce transfusion dependence, and delay progression to acute leukemia.
Aetiology:
With a few exceptions, the exact causes of MDS are unknown.
Some evidence suggests that certain people are born with a tendency to develop MDS. This tendency can be thought of as a switch that is triggered by an external factor. If the external factor cannot be identified, then the disease is referred to as “primary MDS”.
Radiation and chemotherapy for cancer are among the known triggers for the development of MDS. Patients who take chemotherapy drugs or who receive radiation therapy for potentially curable cancers, such as breast or testicular cancers, Hodgkin’s disease and non-Hodgkin’s lymphoma, are at risk of developing MDS for up to 10 years following treatment.
Myelodysplastic syndrome that develops after use of cancer chemotherapy or radiation is called “secondary MDS” and is usually associated with multiple chromosome abnormalities in cells in the bone marrow. This type of MDS often develops rapidly into AML.
Long term exposure to certain environmental or industrial chemicals, such as benzene, can also trigger MDS.
While alcohol consumed on a daily basis may lower red blood cell and platelet counts, alcohol does not cause MDS.
There is insufficient data available to determine if smoking increases the risk of developing MDS. However, it is known that the risk of developing AML is 1.6 times greater for smokers than for non-smokers.
MDS is not inherited. In fact, it is a very rare occasion when family members, including siblings, are diagnosed with MDS.
Cytogenetically, patients with MDS or AML fall into three groups:
•Normal karyotype
•Balanced chromosomal abnormality causing the generation of fusion oncogenes
•Complex karyotypes (usually >3 abnormalities)
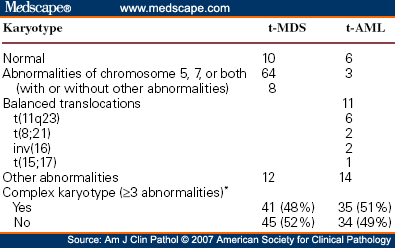
Comparison of cytogenetic abnormalities in therapy-related MDS and therapy-related AML, highlighting chromosome 5/7 abnormalities, balanced translocations, and the prevalence of complex karyotypes.
Clinical features:
Symptoms tend to reflect the most affected cell line and may include pallor, weakness, and fatigue (anemia); fever and infections (neutropenia); and increased bruising, petechiae, epistaxis, and mucosal bleeding (thrombocytopenia).
In addition to reduced numbers of blood cells, the mature blood cells circulating in the blood may not function properly because of dysplasia.
Extramedullary hematopoiesis may occur, leading to hepatomegaly and splenomegaly.
Myelofibrosis is occasionally present at diagnosis or may develop during the course of MDS.
Symptoms may also be referable to other underlying disorders; e.g. in elderly patients with preexisting cardiovascular disorders, anemia from MDS may exacerbate anginal pain.
Diagnosis:
The presence of dysplastic changes in the peripheral blood smear and trilineage dysplasia and hypercellular marrow in the absence of vitamin deficiency is diagnostic of MDS. The presence of typical chromosomal abnormalities supports the diagnosis and contributes to determining the prognosis of MDS.
Myelodysplastic syndrome is suspected in patients (especially the elderly) with refractory anemia, leucopenia, or thrombocytopenia. Cytopenias secondary to congenital disorders, vitamin deficiencies, or drug adverse effects must be ruled out.
Anemia is the most common feature, associated usually with macrocytosis and anisocytosis. With automatic cell counters, these changes are indicated by an increased MCV and RBC distribution width. Punctate basophilia is observed in RBCs.
Some degree of thrombocytopenia is usual; on peripheral smear, platelets vary in size, and some appear hypogranular. Abnormalities such as giant hypogranular platelets and megakaryocyte fragments are present.
The WBC count may be normal, increased, or decreased. Neutrophil cytoplasmic granularity is abnormal, with anisocytosis and variable numbers of granules. Granulation abnormalities vary from an absence of granules to abnormal distribution inside the cytoplasm (Döhle bodies).
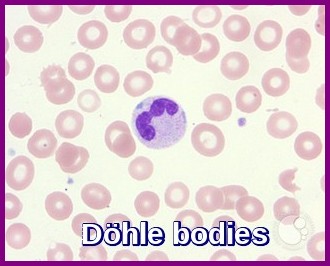
Peripheral smear demonstrating a neutrophil containing Döhle bodies—pale blue cytoplasmic inclusions associated with inflammation, infection, and myelodysplastic syndrome.
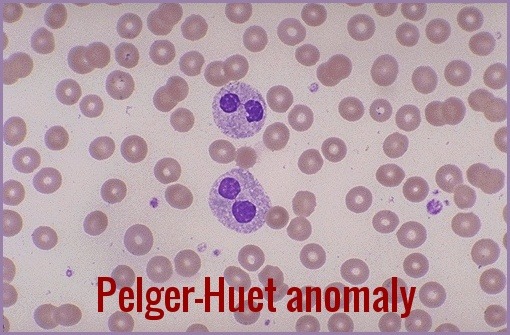
Eosinophils also may have abnormal granularity. Pseudo Pelger-Huët cells (hyposegmented neutrophils) may be seen. Monocytosis is characteristic of the chronic myelomonocytic leukemia (CMML) subgroup, and immature myeloid cells may occur in the less well differentiated subgroups.

Peripheral smear demonstrating Pseudo Pelger–Huët cells—bilobed, hyposegmented neutrophils commonly associated with myelodysplastic syndromes and other marrow disorders.
The cytogenetic pattern is usually abnormal, with one or more clonal cytogenetic abnormalities often involving chromosomes 5 or 7.
In most cases, bone marrow changes include hypercellularity with trilineage dysplastic changes. A small number of patients may have a hypocellular marrow. This often overlaps with aplastic anemia. Increased marrow fibrosis may be confused with other myeloproliferative disorders. Dysplastic changes in RBC lineage (dyserythropoiesis) are characteristic. In the absence of vitamin B12 or folate deficiencies, the bone marrow usually exhibits asynchronous maturation of nuclei and cytoplasm similar to that described in megaloblastic anemias.
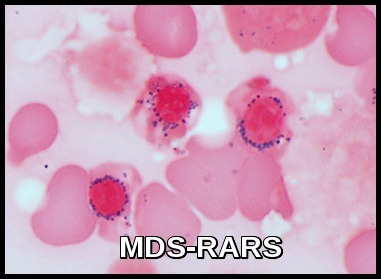
- Other changes include binuclearity or multinuclearity in the erythroid cell precursor cells and the presence of ringed sideroblasts (iron accumulation in the mitochondria). Refractory anemia with ringed sideroblasts (RARS) is one of the MDS types in the French-American-British (FAB) Cooperative Group classification system.
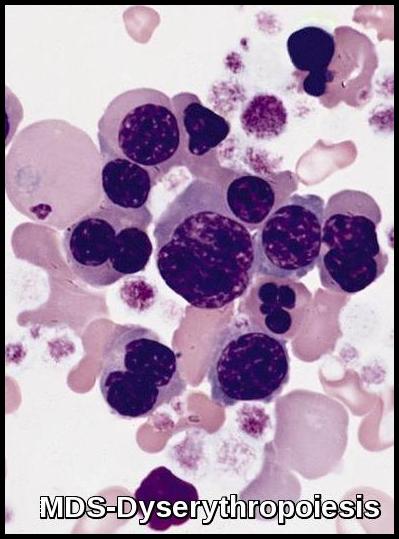
Bone marrow aspirate demonstrating dyserythropoiesis with multinucleated erythroid precursors, nuclear irregularities, and abnormal chromatin patterns, consistent with Myelodysplastic Syndrome.
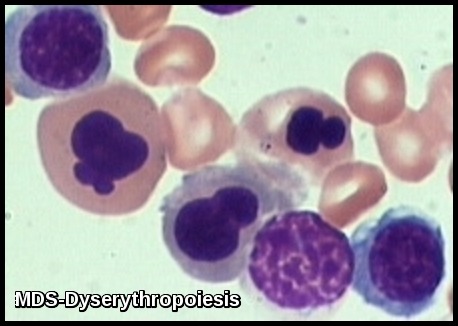
Morphologic features of dyserythropoiesis demonstrating nuclear irregularities, budding, and aberrant chromatin patterns typical of Myelodysplastic Syndrome.
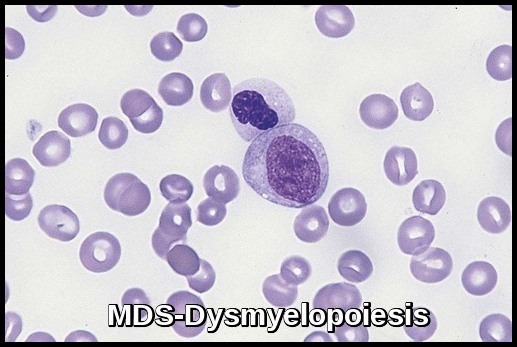
- Dysplastic changes in white blood cell (WBC) lineage (dysmyelopoiesis) involve myeloid hyperplasia with an increased number of myeloblasts and an expanded myelocyte and metamyelocyte population (midstage bulge). This separates it from acute leukemia (absence of mid stage).
- In the FAB classification, the percentage of myeloblasts separates RA (< 5%), RAEB (5-20%), RAEB in transformation (>20, < 30%), and acute myeloid leukemia (AML; >30%). The 2008 update of the WHO classification considers single-lineage dysplasia as a valid criterion for diagnosis of MDS, and Refractory cytopenia with multilineage dysplasia (RCMD) became an official entity in that classification. In 2004, WHO revised the percentage of blasts that defines AML from 30% to 20%; thus, the RAEB in transformation entity has become officially AML.
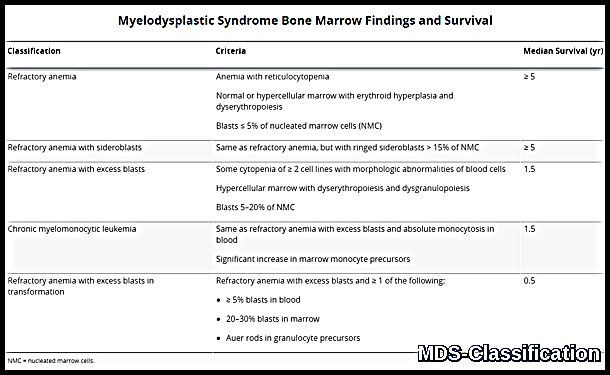
Summary table showing Myelodysplastic Syndrome subtypes with corresponding bone marrow criteria and median survival, including refractory anemia, ring sideroblasts, excess blasts, and CMML.
- Morphologic abnormalities are evident in nuclear-cytoplasm dissociation in maturation and when the pseudo–Pelger forms are also present in bone marrow.
- Dysthrombopoiesis in the platelet production cell lineage consists of micromegakaryocytes (dwarf forms) with poor nuclei lobulation and giant platelets budding off from their cytoplasm.
Cytogenetic studies of the bone marrow cells indicate mutations into clonal cell lines, with abnormal chromosomes in 48-64% in different series. With higher-resolution techniques (e.g. FISH), some practitioners claim a 79% rate of chromosomal abnormalities in patients with primary MDS.
Chromosomal abnormalities are clonal and include 5q-, monosomy 7 (-7) or 7q-, trisomy 8 (+8), and numerous other less frequent abnormalities. Multiple combinations may be present; this indicates a very poor prognosis. A single abnormality, except those involving chromosome 7, usually indicates a good prognosis and survival.
Deletion 5q is a rare form of Myelodysplastic Syndrome (MDS). For many people, it can remain stable for many years causing few symptoms. For others, it may progress rapidly into a different subtype of MDS or transform into acute leukemia. Refractory anemia is very common in Del 5(q) MDS.
Chronic Myelomonocytic Leukemia (CMML):

Peripheral smear demonstrating features of CMML, including persistent monocytosis and dysplastic monocytes with abnormal nuclear morphology and granularity.
Staging:
The International Prognostic Scoring System (IPSS) is a system developed for staging MDS. It was intended for use with the FAB classification system. It rates 3 factors:
1- The percentage of blasts in the bone marrow (scored on a scale from zero to 2)
2- Chromosome abnormalities (scored from zero to 1)
3- The patient’s blood counts. (scored as zero or 0.5)
- Low risk
- Intermediate – 1 risk (Int-1)
- Intermediate – 2 risk (Int-2)
- High risk
Treatment:
The standard care for patients with myelodysplastic syndrome (MDS) and decreased blood counts is constantly changing.
Supportive therapy, including transfusions of the cells that are deficient (i.e. RBCs, platelets), and treatment of infections are the main components of care.
The approach to therapy is based on the International Prognostic Scoring System (IPSS) score, the patient’s age and co-morbidities, and the patient’s expectations and personal goals.
The more toxic and aggressive forms of therapy, such as stem cell transplantation and aggressive chemotherapy, are reserved for younger and fit patients with high-risk disease.
Cytotoxic chemotherapy is used in patients with MDS who have increasing myeloblasts and those who have progressed to acute leukemia. The usual combination treatment is cytarabine plus an anthracycline, which yields a limited response rate of 30-40%. Elderly patients experience high rates of morbidity and mortality with this approach and should consider treatment with the hypomethylating agent azacitidine (Vidaza), which has been shown to relieve symptoms, decrease the rate of transformation to leukemia and the need for transfusions, and probably improve survival compared with either supportive or aggressive therapy and is approved for use in MDS by the US Food and Drug Administration (FDA).
Deoxyazacitidine (Decitabine), another hypomethylating agent, is sometimes effective, even in patients who do not respond to azacitidine.
Lenalidomide (Revlimid) is active in patients with MDS categorized as low risk or intermediate risk–1 according to the International Prognostic Scoring System (IPSS).
Lenalidomide is the drug of choice in MDS with 5q deletion syndrome. In particular, patients with the karyotype characterized by deletion 5q31 show the best response. List et al reported an erythroid response in 76% of these patients, with 67% no longer requiring transfusions; 73% of their 148 patients had a cytogenetic response, 45% had a complete cytogenetic remission, and 36% achieving a normal bone marrow histologically.
In some patients, erythropoietin to support RBC needs, granulocyte colony-stimulating factor (G-CSF) to manage severe symptomatic granulocytopenia, and, when available, thrombopoietin for severe thrombocytopenia can serve as important hematopoietic support but have not increased survival.
Allogeneic stem cell transplantation is the treatment of choice for young patients, and nonablative allogeneic bone marrow transplantations are now being studied for patients > 50 years. Response of MDS to chemotherapy, typically regimens similar to those used for AML, is similar to that of AML after age and karyotype are considered.
Some cases of MDS have an autoimmune process underlying the pancytopenia and respond to immunosuppressive therapy. However, identifying these cases is problematic. A portion of these cases represent an overlap between aplastic anemia (AA), paroxysmal nocturnal hemoglobinuria (PNH), and MDS. These patients are usually younger and may have hypoplastic bone marrow with dysplasia and cytogenetic abnormalities (separate from AA). In addition, they may have a small clone of PNH cells, but these typically constitute less than 3% of cells and require a special sensitive flow cytometry to be detected.
Although treatment of symptoms improves quality of life in MDS, these measures are temporary. More long-term measures are necessary to stimulate the patient’s bone marrow production of mature blood cells.
Practitioners are encouraged to refer patients for participation in clinical trials at academic centers and the MDS Centers of Excellence.
Questions and Answers:
What are the early signs and symptoms of Myelodysplastic Syndrome (MDS)?
Early symptoms are often subtle and relate to cytopenias. Patients may experience fatigue and pallor from anemia, recurrent infections from neutropenia, or easy bruising, petechiae, or bleeding due to thrombocytopenia. Some cases are identified incidentally on routine blood tests showing unexplained cytopenias or macrocytosis.
How is Myelodysplastic Syndrome diagnosed?
Diagnosis is based on persistent cytopenias, dysplastic features on peripheral smear, and bone marrow aspirate/biopsy showing dysplasia in one or more lineages, abnormal blast percentages, or ring sideroblasts. Cytogenetics, molecular panels, and prognostic scoring systems such as IPSS or IPSS-R support accurate diagnosis and risk classification.
What causes Myelodysplastic Syndrome?
MDS arises from clonal mutations in hematopoietic stem cells. Causes include age-related genetic changes, prior chemotherapy or radiotherapy (therapy-related MDS), environmental toxins such as benzene, and inherited predisposition syndromes. These mutations disrupt normal hematopoiesis, leading to ineffective cell production and dysplasia.
What is the risk of progression from MDS to acute myeloid leukemia (AML)?
The risk varies by MDS subtype and prognostic score, with higher-risk categories showing substantially greater transformation rates. About 25–30% of patients with MDS may progress to AML, particularly those with excess blasts or adverse cytogenetic and molecular features.
What are the treatment options for Myelodysplastic Syndrome?
Management is risk-adapted and includes supportive measures (transfusions, erythropoiesis-stimulating agents), hypomethylating agents such as azacitidine or decitabine, lenalidomide for del(5q) disease, iron chelation when indicated, and infection management. Allogeneic stem cell transplantation is the only curative therapy for eligible patients.
How is prognosis determined in patients with MDS?
Prognosis is assessed using systems such as the IPSS, IPSS-R, WHO classification, or ICC. Variables include bone marrow blast percentage, cytopenia severity, cytogenetic abnormalities, and presence of high-risk mutations. Survival ranges from years in low-risk disease to rapid progression in high-risk categories.
What is the role of genetic and molecular testing in MDS?
NGS-based mutation profiling identifies key genes such as SF3B1, TET2, ASXL1, RUNX1, and TP53. These mutations refine diagnosis, guide therapy selection, and significantly influence prognosis. For example, SF3B1 mutation predicts favorable outcomes in MDS with ring sideroblasts, whereas TP53 mutations are associated with poor response and aggressive disease.
References:
Besa EC. Myelodysplastic syndromes (refractory anemia): A perspective of the biologic, clinical, and therapeutic issues. Med Clin North Am. 1992;76(3):599–617.
Bejar R, Steensma DP. Recent developments in myelodysplastic syndromes. Blood. 2014;124(18):2793–2803.
Emmanuel C Besa, MD. Myelodysplastic Syndrome: Background, Pathophysiology, Etiology. Medscape. Accessed September 2016. https://emedicine.medscape.com/article/207347-overview
Germing U, Kobbe G, Haas R, Gattermann N. Myelodysplastic syndromes: diagnosis, prognosis, and treatment. Dtsch Arztebl Int. 2013;110(46):783–790.
Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088.
Greenberg PL, et al. Revised International Prognostic Scoring System (IPSS-R) for Myelodysplastic Syndromes. Blood. 2012;120(12):2454–2465. doi:10.1182/blood-2012-03-420489
Haase D. Cytogenetic and molecular genetic pathways in the evolution of myelodysplastic syndromes. Haematologica. 2020;105(6):1456–1468. doi:10.3324/haematol.2019.232116
Khoury JD, et al. The 2022 WHO Classification of Myeloid Neoplasms and Acute Leukemias: Key updates and diagnostic principles. Blood. 2022;140(11):1200–1228. doi:10.1182/blood.2022015850
List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–1465.
List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549–557.
Michael E Rytting, MD. Myelodysplastic Syndrome – Hematology and Oncology – Merck Manuals Professional Edition. Accessed October 2014. https://www.merckmanuals.com/professional/hematology-and-oncology/leukemias/myelodysplastic-syndrome
National Cancer Institute (NCI). Myelodysplastic Syndromes Treatment (PDQ®) – Health Professional Version. https://www.cancer.gov/types/myeloproliferative/hp/myelodysplastic-treatment-pdq
National Institutes of Health (NIH) / MedlinePlus. Myelodysplastic Syndromes (MDS). https://medlineplus.gov/myelodysplasticsyndromes.html
National Organization for Rare Disorders (NORD). Myelodysplastic Syndromes – Overview and Management. https://rarediseases.org/rare-diseases/myelodysplastic-syndromes
Patnaik MM, Tefferi A. Chronic Myelomonocytic Leukemia: 2022 Update on Diagnosis, Risk Stratification and Management. Am J Hematol. 2022. doi:10.1002/ajh.26455
Patnaik MM, Tefferi A. Chronic Myelomonocytic Leukemia (CMML). Medscape. Updated 2024. https://emedicine.medscape.com/article/198320-overview
Savona MR, et al. Chronic myelomonocytic leukemia: 2023 update on diagnosis, prognosis, and treatment. Blood Rev. 2023;58:101015. doi:10.1016/j.blre.2023.101015
Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20(10):2429–2440.
Keywords:
myelodysplastic syndrome, MDS, myelodysplastic syndromes, MDS classification, MDS subtypes, bone marrow failure MDS, MDS causes, MDS diagnosis, MDS symptoms, MDS cytogenetics, MDS genetic mutations, MDS dysplasia, refractory anemia MDS, ring sideroblasts MDS, MDS-RS, dyserythropoiesis, dysmyelopoiesis, micromegakaryocytes, pseudo Pelger-Huët cells, Döhle bodies MDS, MDS blood film findings, MDS bone marrow biopsy, IPSS MDS, IPSS-R MDS, MDS prognosis, MDS survival rate, MDS risk factors, MDS to AML transformation, AML progression risk, MDS pathophysiology, clonal hematopoiesis MDS, MDS molecular markers, MDS TP53 mutation, MDS SF3B1 mutation, CMML, chronic myelomonocytic leukemia, CMML diagnosis, CMML blood film, CMML treatment, hypomethylating agents MDS, azacitidine MDS, decitabine MDS, lenalidomide MDS, MDS 5q deletion, MDS treatment options, MDS supportive care, MDS transfusion dependence, iron overload MDS, erythropoiesis-stimulating agents MDS, ESA therapy MDS, MDS stem cell transplant, allogeneic stem cell transplant MDS, hematology MDS, blood cancer MDS, early MDS signs, leukaemia care MDS, myeloid neoplasms, WHO 2022 MDS classification, Ask Hematologist, Dr Moustafa Abdou.






hello team
kindly let me know about MDS -del 20 and treatment
Hello,
Thank you for your comment. Myelodysplastic patients with del 20q have a lower incidence of anemia, lower incidence of progression to acute myeloid leukemia, and more prolonged survival. Treatment of MDS is mainly supportive. Myelodysplastic patients with excess blasts in their bone marrow might benefit from some chemotherapeutic agents like Azacitidine (Vidaza®). You can read more about this disorder through this link: https://askhematologist.com/myelodysplastic-syndrome/
Best wishes,
Author
Is their a treatment for. It
Hi Maria,
Thank you for reaching out.
Yes, myelodysplastic syndrome (MDS) can be treated with various approaches such as medications (e.g., lenalidomide, azacitidine), blood transfusions, growth factors, and potentially stem cell transplantation, depending on the specific case and severity.
Best wishes,
Dr. M. Abdou