Waldenström’s Macroglobulinemia
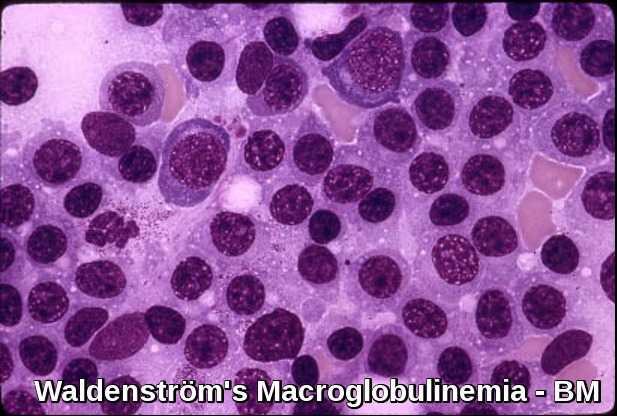
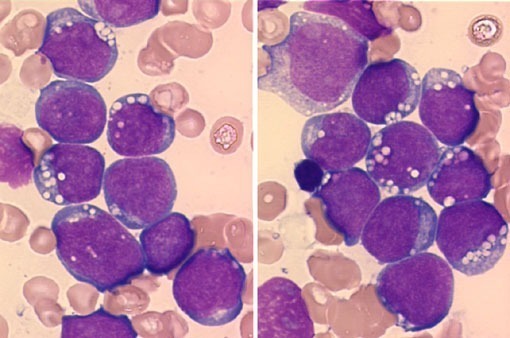
Bone marrow infiltration in Waldenström’s Macroglobulinemia showing lymphoplasmacytic lymphoma morphology.
Waldenström’s macroglobulinemia (WM), a subtype of lymphoplasmacytic lymphoma, is an indolent B-cell malignancy characterized by clonal proliferation of small lymphocytes with plasmacytoid differentiation in the bone marrow and excessive production of monoclonal IgM. This rare hematologic neoplasm behaves more like a lymphomatous disorder than a plasma-cell dyscrasia and is classified among the malignant monoclonal gammopathies. The marked overproduction of IgM leads to a range of clinical manifestations, including hyperviscosity, neuropathy, and cytopenias. Although the exact etiology of Waldenström’s macroglobulinemia remains unknown, it predominantly affects older adults, with men more frequently diagnosed than women.

Serum protein electrophoresis in Waldenström’s Macroglobulinemia demonstrating the characteristic monoclonal IgM M-protein spike.
Clinical features:
Waldenström’s macroglobulinemia is a disease of the elderly (median age is 65 years). After myeloma, WM is the 2nd most common malignant disorder associated with a Monoclonal Gammopathy.
The disease usually presents with symptoms of Hyperviscosity. Symptoms of hyperviscosity include fatigue, weakness, spontaneous bleeding from mucous membranes, visual disturbances due to retinopathy, and neurologic symptoms ranging from headaches and vertigo to seizures and coma. Normal plasma viscosity is between 1.4 and 1.8 centipoise while symptoms from hyperviscosity typically occur when plasma viscosity is greater than 4 centipoise (about 4 times more viscous than water) and require emergency treatment.
weight loss is also common.
There may be hepatosplenomegaly or lymphadenopathy (cf. myeloma).
Bony lesions are not usually seen in Waldenström’s macroglobulinemia (cf. myeloma).
Investigations:
- Anemia and a high ESR are usual.
- The leucocyte count and platelet count are often normal, though malignant lymphoid cells may be seen.
- The bone marrow shows diffuse infiltration by plasmacytoid lymphocytes. Flow cytometry results show B-cell features with surface expression of IgM and B-cell differentiation markers. In practice, a sIgM+ CD5– CD10– CD19+ CD20+ CD23–immunophenotype in association with a nonparatrabecular pattern of bone marrow infiltration is diagnostic of Waldenström’s macroglobulinemia.
- The serum IgM is usually very high and other immunoglobulins may be low.
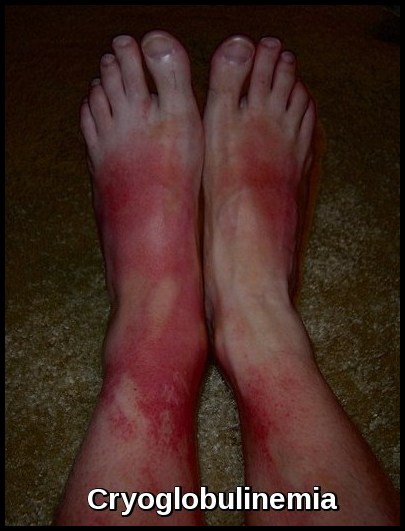
- Cryoglobulins and Bence-Jones proteins may be present.
- Plasma viscosity is usually very high.
- Renal impairment is unusual (cf. myeloma).
A particular mutation in the MYD88 gene is found in more than 90 percent of people with Waldenström macroglobulinemia. The mutation involved in this condition changes a single protein building block (amino acid) in the MyD88 protein, replacing the amino acid leucine with the amino acid proline at position 265 (written as Leu265Pro or L265P). The mutation is acquired during a person’s lifetime and is present only in the abnormal white blood cells. This type of genetic change, called a somatic mutation, is not inherited. Waldenström macroglobulinemia is thought to result from multiple genetic changes, including the MYD88 gene mutation.
The altered MyD88 protein is constantly functioning (overactive). It stimulates the signaling molecules that activate nuclear factor-kappa-B, even without signals from outside the cell. Researchers suggest that abnormally active nuclear factor-kappa-B allows survival of abnormal cells that should undergo apoptosis, which may contribute to the accumulation of lymphoplasmacytic cells in Waldenström macroglobulinemia.
Treatment:
Indications for initiating active treatment for Waldenström’s macroglobulinemia include clinical evidence of adverse effects of the paraprotein (hyperviscosity with neurologic or ocular disturbance, peripheral neuropathy, amyloidosis, symptomatic cryoglobulinemia, cytopenias), disease progression (including progressive anemia), or development of constitutional symptoms.
No treatment is indicated for asymptomatic disease.
Treatment with oral alkylating drugs e.g. Chlorambucil may be indicated for palliation, but bone marrow toxicity can occur.
Corticosteroids may be effective in reducing tumor load.
Rituximab can reduce tumor burden without suppressing normal hematopoiesis. However, during the first several months, IgM levels may increase, requiring plasma exchange.
Combinations of Rituximab with Cyclophosphamide/Dexamethasone, Bendamustine, or Bortezomib/Dexamethasone provide durable responses and are indicated when treatment is required.
Nucleoside analogs (Fludarabine and 2-chlorodeoxyadenosine) produce responses in large numbers of newly diagnosed patients but have been associated with a high risk of myelodysplasia and myeloid leukemia.
New monoclonal antibodies (ofatumumab), second-generation proteasome inhibitors (carfilzomib), immunomodulatory agents (thalidomide, pomalidomide, or lenalidomide), and Bruton’s tyrosine kinase inhibitors (Ibrutinib) are promising and may expand future treatment options.
Bruton’s tyrosine kinase (BTK) plays a key role in signaling pathways for the survival of WM clone. BTK inhibition has changed the treatment landscape of the disease. Ibrutinib has resulted in deep and durable responses both as an upfront and salvage treatment with a manageable toxicity profile. However, the need for fewer off-target effects and deeper responses has resulted in the clinical development of second-generation BTK inhibitors. Zanubrutinib has resulted in clinically meaningful antitumor activity, including deep and durable responses, with a low discontinuation rate due to treatment-related toxicities. Cardiovascular adverse events seem to be milder compared with ibrutinib. Interestingly, the efficacy of zanubrutinib in WM is significant both for MYD88L265P and MYD88WT patients.
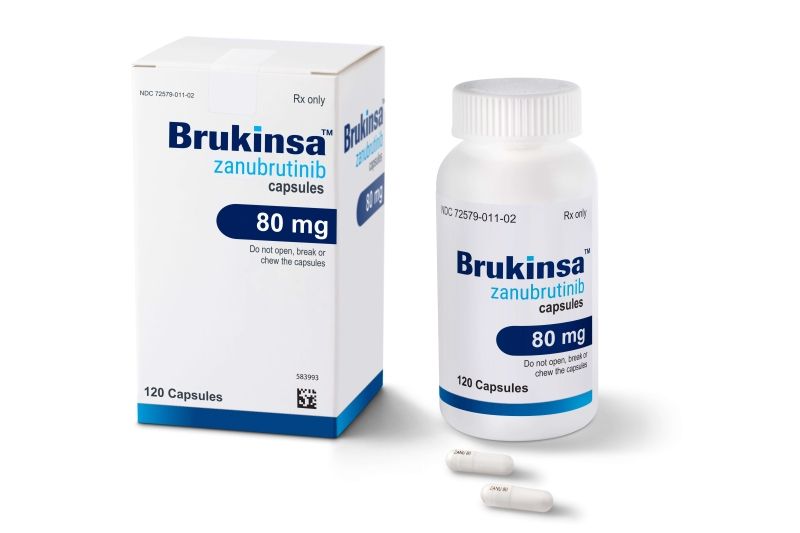
Brukinsa (zanubrutinib) 80 mg capsules commonly used in the treatment of Waldenström’s Macroglobulinemia.
Plasmapheresis may be useful for hyperviscosity.
Autologous stem cell transplantation may be considered in young patients with chemosensitive disease and in newly diagnosed patients with very high-risk features.
Prognosis:
The prognosis of Waldenström’s Macroglobulinemia is highly variable, with a median overall survival generally ranging from 7 to 10 years, though many patients experience significantly longer survival with modern therapies. Outcomes are closely linked to disease burden and host factors, and validated prognostic systems consistently highlight age over 60 years, anemia, thrombocytopenia, elevated β2-microglobulin, and the presence of cryoglobulinemia as predictors of shorter survival. Additional adverse factors include high IgM levels, significant marrow infiltration, constitutional symptoms, and treatment-refractory disease. With the advent of targeted agents such as BTK inhibitors, survival trends continue to improve, particularly in patients with favorable genomic profiles such as MYD88 L265P without CXCR4 mutations.
Questions and Answers:
What is Waldenström’s Macroglobulinemia and how does it differ from myeloma?
Waldenström’s Macroglobulinemia is a type of lymphoplasmacytic lymphoma characterized by clonal B-cell proliferation and excess monoclonal IgM. Unlike multiple myeloma, WM behaves more like a lymphoma, typically lacks lytic bone disease, and involves small lymphocytes with plasmacytoid differentiation rather than mature plasma cells.
What symptoms are most commonly associated with Waldenström’s Macroglobulinemia?
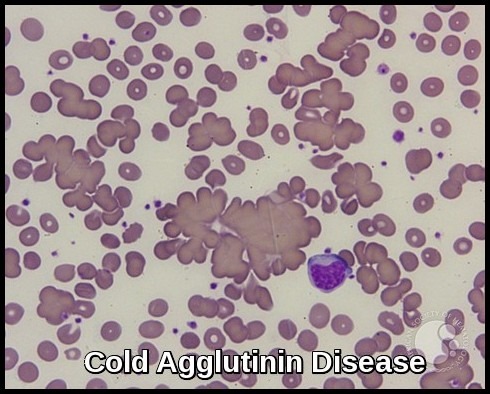
Patients may present with fatigue from anemia, neuropathy, recurrent infections, bruising, hyperviscosity symptoms such as blurred vision and headaches, or manifestations of cryoglobulinemia and cold agglutinin disease. Some remain asymptomatic at diagnosis.
How is Waldenström’s Macroglobulinemia diagnosed?
Diagnosis requires bone marrow infiltration by lymphoplasmacytic lymphoma and detection of a monoclonal IgM component on SPEP or immunofixation. Work-up includes full blood count, serum viscosity, β2-microglobulin, MYD88 L265P mutation testing, and imaging when clinically indicated.
What causes elevated IgM levels in Waldenström’s Macroglobulinemia?
The abnormal B-cell clone produces large amounts of monoclonal IgM, leading to hyperviscosity, impaired circulation, neuropathy, and various immune-mediated complications including cryoglobulinemia and cold agglutinin hemolysis.
When should treatment be started for Waldenström’s Macroglobulinemia?
Treatment is indicated only in symptomatic or progressive disease—such as anemia, hyperviscosity syndrome, neuropathy, bulky lymphadenopathy, organomegaly, constitutional symptoms, cryoglobulinemia, or significant IgM-related complications. Asymptomatic patients are observed.
What are the most effective treatments for Waldenström’s Macroglobulinemia?
Current first-line options include BTK inhibitors such as zanubrutinib, ibrutinib, and acalabrutinib, as well as rituximab-based combinations. Therapy selection depends on age, comorbidities, genomic profile (MYD88/CXCR4), and disease complications such as hyperviscosity or neuropathy.
What is the role of plasmapheresis in Waldenström’s Macroglobulinemia?
Plasmapheresis rapidly reduces IgM levels and is recommended for symptomatic hyperviscosity, severe neuropathy, cryoglobulinemia, or before rituximab initiation in patients with very high IgM to prevent IgM flare.
Is Waldenström’s Macroglobulinemia curable?
WM is currently incurable but highly manageable, with many patients achieving long-term disease control using targeted therapy. Survival continues to improve with modern treatments, especially BTK inhibitors.
What factors predict prognosis in Waldenström’s Macroglobulinemia?
Poor prognostic factors include age over 60, anemia, elevated β2-microglobulin, thrombocytopenia, cryoglobulinemia, high IgM levels, CXCR4 mutations, and significant marrow infiltration. Patients with MYD88 L265P mutation generally respond better to BTK inhibitors.
Can Waldenström’s Macroglobulinemia transform into a more aggressive lymphoma?
Rarely, WM can transform into diffuse large B-cell lymphoma (DLBCL). Transformation is suggested by rapid clinical deterioration, rising LDH, new bulky disease, and high-grade features on biopsy.
References:
Oza A, Rajkumar SV. Waldenström macroglobulinemia: prognosis and management. Blood Cancer J. 2015;5(3):e296. doi: 10.1038/bcj.2015.28.
James R. Berenson, MD. Macroglobulinemia – Hematology and Oncology – Merck Manuals Professional Edition. Available at: https://www.merckmanuals.com/professional/hematology-and-oncology/plasma-cell-disorders/macroglobulinemia. Accessed September 2016.
Vogt RF, Marti GE. Overview of monoclonal gammopathies of undetermined significance. Br J Haematol. 2007;139(5):687–689.
MedlinePlus Genetics. MYD88 Gene. Available at: https://medlineplus.gov/genetics/gene/myd88/
Ntanasis-Stathopoulos I, Gavriatopoulou M, Fotiou D, Dimopoulos MA. Current and novel BTK inhibitors in Waldenström’s macroglobulinemia. Ther Adv Hematol. 2021;12:2040620721989586. doi: 10.1177/2040620721989586.
Treon SP, Xu L, Liu X, et al. MYD88 L265P somatic mutation in Waldenström’s Macroglobulinemia. N Engl J Med. 2012;367:826–833.
Buske C, Leblond V. How I treat Waldenström’s Macroglobulinemia. Blood. 2013;122(26):4180–4185. doi:10.1182/blood-2013-06-510982.
Keywords:
Waldenström’s macroglobulinemia, lymphoplasmacytic lymphoma, WM symptoms, WM diagnosis, WM treatment options, IgM monoclonal gammopathy, monoclonal IgM spike, IgM hyperviscosity syndrome, cryoglobulinemia WM, cold agglutinin disease WM, peripheral neuropathy WM, bone marrow lymphoplasmacytic infiltration, MYD88 L265P mutation, CXCR4 mutation WM, serum protein electrophoresis IgM spike, immunofixation IgM monoclonal band, BTK inhibitors WM, zanubrutinib for Waldenström’s macroglobulinemia, ibrutinib WM therapy, acalabrutinib WM therapy, rituximab WM, bendamustine rituximab WM, plasmapheresis for hyperviscosity, WM prognosis factors, WM survival rates, WM clinical features, WM treatment guidelines, IgM-related complications, WM vs multiple myeloma, WM differential diagnosis, malignant monoclonal gammopathies, Ask Hematologist, Dr Moustafa Abdou


Request Online Consultation With Dr M Abdou
Fee: US$100
Secure payment via PayPal (credit and debit cards accepted)
Pay Now


Hi,
iI am a gp based in Germany. I have been trying hard to understand the difference between Waldenstroms macroglobulinemia and a IgM Myeloma…
Hi Pawel,
Thank you for your comment.
Waldenström’s macroglobulinaemia (WM) and IgM myeloma can appear similar but have distinct features. WM is a lymphoplasmacytic lymphoma involving bone marrow infiltration and an IgM monoclonal protein, typically associated with the MYD88 L265P mutation and CD20 positivity. It often presents with lymphadenopathy, splenomegaly, or hyperviscosity symptoms, and rarely causes lytic bone lesions. In contrast, IgM myeloma is a rare plasma cell malignancy that produces IgM, is MYD88-negative, and is associated with CRAB features (hypercalcaemia, renal impairment, anemia, and bone lesions). It shows CD138 positivity and may have myeloma-specific cytogenetic abnormalities such as t(11;14). Key tests to differentiate include bone marrow immunophenotyping, MYD88 mutation testing, and cytogenetics.
BW,
Dr M Abdou