Thalassemias

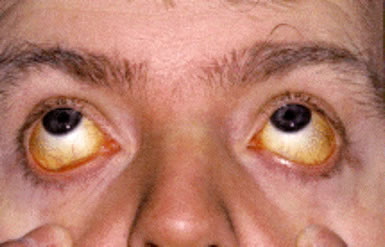
Classic thalassemia major with thalassemic facies and “hair-on-end” appearance on skull X-ray caused by chronic bone marrow expansion.
What are Thalassemias?

Organization of the alpha and beta globin gene clusters on chromosomes 16 and 11 explaining the genetic basis of alpha and beta thalassemia.
Thalassemias are a group of inherited blood disorders characterized by defective production of hemoglobin, the oxygen-carrying protein within red blood cells. This abnormal hemoglobin synthesis leads to premature destruction of red blood cells, resulting in chronic hemolytic anemia of varying severity. The underlying defect in thalassemias arises from mutations in one or more of the globin genes, causing reduced or absent synthesis of specific globin chains. This creates a pathological imbalance between alpha (α) and beta (β) globin chains, leading to globin chain precipitation, ineffective erythropoiesis within the bone marrow, and increased peripheral hemolysis. Structurally, the normal hemoglobin molecule is a tetramer composed of four polypeptide globin chains—typically two alpha chains (each 141 amino acids long) and two beta chains (each 146 amino acids long). Each globin chain is bound to an iron-containing heme group, which enables hemoglobin to bind and transport oxygen efficiently throughout the body via oxygenation rather than oxidation.
Clinical syndromes of the ß thalassemias:
Thalassemia major (disease):
- This occurs when both ß genes are Thalassemic.
- Anemia develops at about 2 months of age (after HbF⇒HbA switch).
- Untreated there is a failure to thrive and physical retardation.
- The child develops bossing of the skull, ‘Mongoloid’ facies and hepatosplenomegaly.
- Plain skull X-ray shows the classical ‘hair on end‘ appearance. The maxilla may overgrow with a prominence of the upper incisors, and separation of the orbit. These produce the characteristic thalassemia facies in thalassemia major.
- Death usually occurs in childhood if untreated.
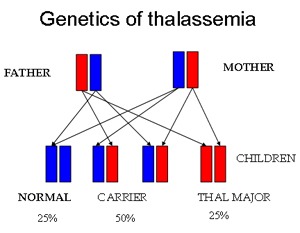
Genetic inheritance of thalassemia showing the autosomal recessive transmission with 25% normal, 50% carrier, and 25% thalassemia major risk per pregnancy.
Thalassemia minor (trait):
- Affected individuals have only one affected ß gene.
- Anemia is either not present or very mild.
Thalassemia intermedia:
This is a heterogeneous group of disorders in which both ß genes and sometimes one or more ∝ genes are affected but the disease process is relatively mild with no transfusion requirement and little physical retardation.
Investigations:

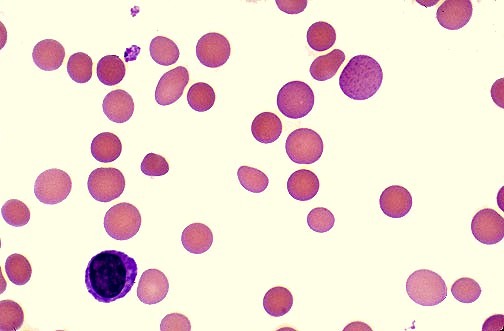
Peripheral blood smear in β-thalassemia major showing severe microcytic hypochromic anemia with target cells, anisopoikilocytosis, and nucleated red blood cells.
- The Hb is variably reduced.
- The RBCs are invariably microcytic in all types of the disorder.
- In the disease state target cells, stippled cells and nucleated RBCs are seen in the blood film.
- In ß thalassemia trait, HbF is usually normal and HbA2 is slightly raised (5%). In disease, there is increased HbF and HbA2. HbA may be absent in homozygous ß thalassemia.
Clinical syndromes of the ∝ thalassemias:
Silent ∝ thalassemia:
Only one of the genes is affected and a slight reduction in the MCV is the only abnormality.
Thalassemia trait:
Two or three ∝ genes are affected and individuals may be asymptomatic or mildly anemic.
Hb Bart’s hydrops fetalis:
All four ∝ genes are involved, leading to inadequate HbF production with intrauterine death.
Investigations:
- The MCV is reduced and HbH (ß4 tetramers inclusion bodies) may be seen in RBCs.
- Electrophoresis shows the presence of HbH (not in silent form) and there may be a small amount of Hb Barts, especially at birth.
- HbA is not raised.
- In hydrops fetalis there is no HbA, HbA2 or HbF; only Hb Barts and some embryonic hemoglobins.
Treatment:
- In ∝ and ß thalassemia trait problems usually only arise with infections or during pregnancy. Folic acid is usually given throughout pregnancy.
- Nutritional supplements, in the form of folic acid supplements, and monitoring of B12 levels are important, as these nutrients are key components to making healthy blood cells.
- It is important to prevent patients from being given iron supplements because of the low MCV.
- In ß thalassemia disease, patients often require frequent blood transfusions, possibly every few weeks. Over time, blood transfusions cause a buildup of iron. Iron chelation with deferasirox (Exjade) or subcutaneous desferrioxamine (Desferal) can help remove the excess iron.
- Some patients with ß thalassemia intermedia may need treatment for iron overload with deferasirox. Although most people with this condition don’t need the blood transfusions that often cause iron overload, people with ß thalassemia intermedia may have increased digestive absorption of iron, leading to an excess of iron.
- Splenectomy is required if the transfusion requirement increases.
- Bone marrow and stem cell transplant from a compatible related donor is the only treatment that can cure thalassemia. It is the most effective treatment. Compatible means that the donor has the same types of proteins, called human leukocyte antigens (HLA), on the surface of their cells as the person who will be receiving the transplant. Bone marrow transplant from a compatible brother or sister offers the best chance at a cure. Most patients with thalassemia, however, lack a suitable sibling donor. A bone marrow transplant is done in the hospital. Within 1 month, the transplanted bone marrow stem cells will start to make new, healthy blood cells. Given the high risks of a bone marrow transplant, it is not routinely recommended for those with mild or moderate thalassemia.
Prenatal testing:
Testing can be done before a baby is born to find out if it has thalassemia and determine how severe it may be.
Tests used to diagnose thalassemia in fetuses include:
- Chorionic villus sampling: this test is usually done around the 11th week of pregnancy and involves removing a tiny piece of the placenta for molecular analysis.
- Amniocentesis: This test is usually done around the 16th week of pregnancy and involves taking a sample of the amniotic fluid.
Summary:
Thalassemias are a group of inherited hemoglobin disorders caused by defective synthesis of alpha or beta globin chains, resulting in chronic hemolytic anemia, ineffective erythropoiesis, and multisystem complications. They are classified into alpha thalassemia and beta thalassemia, with clinical severity ranging from silent carrier states to life-threatening transfusion-dependent thalassemia major. Advances in molecular diagnostics allow precise genetic classification, carrier screening, and prenatal diagnosis, while modern management includes regular blood transfusions, iron chelation therapy, splenectomy in selected cases, and curative stem cell transplantation. Early diagnosis and multidisciplinary care are essential to prevent iron overload, growth failure, skeletal deformities, endocrine dysfunction, and cardiac complications, thereby significantly improving survival and quality of life in patients with thalassemia.
Questions and Answers:
What are thalassemias?
Thalassemias are inherited blood disorders caused by reduced or absent synthesis of alpha or beta globin chains, leading to chronic hemolytic anemia, ineffective erythropoiesis, and variable clinical severity.
What is the difference between alpha and beta thalassemia?
Alpha thalassemia results from deletions or mutations of alpha globin genes on chromosome 16, while beta thalassemia is caused by defects in the beta globin gene on chromosome 11, affecting hemoglobin production differently.
Which is more common, alpha thalassemia or beta thalassemia?
Globally, alpha thalassemia is more common than beta thalassemia due to the high prevalence of alpha globin gene deletions in Southeast Asia, Africa, and the Middle East, while beta thalassemia is more prevalent in the Mediterranean region, South Asia, and the Middle East.
What causes thalassemia major?
Thalassemia major occurs when a child inherits two severely defective globin genes, leading to profound anemia that requires lifelong regular blood transfusions for survival.
What are the facial features seen in thalassemia major?
Thalassemia major causes characteristic thalassemic facies, including frontal bossing, maxillary overgrowth, depressed nasal bridge, and malar prominence due to chronic bone marrow expansion.
What is the hair-on-end appearance on skull X-ray in thalassemia?
The hair-on-end appearance refers to perpendicular trabeculations seen on skull X-ray caused by marked bone marrow hyperplasia in severe chronic hemolytic anemia such as thalassemia major.
How is thalassemia inherited?
Thalassemia is inherited in an autosomal recessive pattern, meaning both parents must be carriers for a child to be affected with thalassemia major.
What is the genetic risk when both parents are carriers of thalassemia?
When both parents are carriers, each pregnancy carries a 25% chance of having an affected child, a 50% chance of having a carrier child, and a 25% chance of having a completely normal child.
What does the peripheral blood smear show in beta thalassemia major?
The blood smear shows severe microcytosis, hypochromia, target cells, anisopoikilocytosis, and numerous nucleated red blood cells reflecting ineffective erythropoiesis.
How is thalassemia diagnosed?
Thalassemia is diagnosed using complete blood count, peripheral blood smear, hemoglobin electrophoresis, high-performance liquid chromatography, and molecular genetic testing.
What complications occur in untreated thalassemia major?
Untreated thalassemia major leads to severe anemia, growth failure, skeletal deformities, iron overload, cardiac disease, endocrine failure, liver fibrosis, and early mortality.
What is iron overload in thalassemia?
Iron overload results from repeated blood transfusions and increased intestinal absorption of iron, leading to toxic deposition in the heart, liver, and endocrine organs.
How is iron overload treated in thalassemia?
Iron overload is treated with iron chelation therapy using agents such as deferoxamine, deferasirox, or deferiprone to prevent organ damage.
Is thalassemia curable?
The only established curative treatment for thalassemia is hematopoietic stem cell transplantation in selected patients with suitable donors.
Can thalassemia be detected during pregnancy?
Yes, thalassemia can be detected prenatally through chorionic villus sampling or amniocentesis with molecular genetic analysis.
Which populations are most affected by thalassemia?
Thalassemia is most common in people from the Mediterranean region, Middle East, South Asia, Southeast Asia, and Africa.
What is thalassemia trait?
Thalassemia trait refers to the carrier state in which individuals have mild microcytic anemia but are usually asymptomatic.
Why does thalassemia cause bone deformities?
Bone deformities occur due to chronic bone marrow expansion in response to ineffective erythropoiesis, leading to thinning of cortical bone and skeletal remodeling.
What is ineffective erythropoiesis in thalassemia?
Ineffective erythropoiesis refers to the failure of red blood cell precursors to mature properly in the bone marrow, causing severe anemia despite increased marrow activity.
How does thalassemia affect life expectancy?
With modern transfusion programs, iron chelation, and multidisciplinary care, many patients with thalassemia major now survive well into adulthood.
Why is genetic screening important in thalassemia?
Genetic screening helps identify carriers, prevent affected births through counseling, and enables early diagnosis and management in affected children.
References:
Borgna-Pignatti C, Galanello R. Thalassemias and related disorders: Quantitative disorders of hemoglobin synthesis. In: Greer JP, et al., editors. Wintrobe’s Clinical Hematology. 12th ed. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 1083–1131.
Weatherall DJ. The thalassemias: Disorders of globin synthesis. In: Kaushansky K, et al., editors. Williams Hematology. 8th ed. New York: McGraw-Hill; 2010. p. 675–707.
Hillman R, et al. Thalassemia. In: Hematology in Clinical Practice. 5th ed. New York: McGraw-Hill; 2011. p. 65–78.
Cooley T, Lee PA. A series of cases of splenomegaly in children with anemia and peculiar bone changes. Transactions of the American Pediatric Society. 1925;37:29–30.
Flint J, et al. The population genetics of the haemoglobinopathies. Baillière’s Clinical Haematology. 1993;6:215–362.
Thalassemias: Management and Treatment. Cleveland Clinic. Available at:
https://my.clevelandclinic.org/health/diseases/14508-thalassemias/management-and-treatment
National Heart, Lung, and Blood Institute (NHLBI). What Are Thalassemias? Available at: https://www.nhlbi.nih.gov/health-topics/thalassemia
National Heart, Lung, and Blood Institute (NHLBI). What Causes Thalassemias? 3 July 2012. Archived 26 August 2016. Available at:
https://www.nhlbi.nih.gov/health/health-topics/topics/thalassemia/causes (Retrieved 5 September 2016).
Khan A, Rehman AU. Laboratory evaluation of beta thalassemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. Updated 30 August 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK585044/
Keywords:
thalassemia, thalassemias, alpha thalassemia, beta thalassemia, thalassemia major, thalassemia intermedia, thalassemia trait, hemoglobinopathy, inherited anemia, globin gene mutations, alpha globin gene, beta globin gene, chromosome 11 beta globin, chromosome 16 alpha globin, thalassemia genetics, autosomal recessive inheritance, thalassemia carrier, prenatal diagnosis thalassemia, hemoglobin electrophoresis thalassemia, HPLC thalassemia, microcytic hypochromic anemia, target cells thalassemia, nucleated red cells thalassemia, hair on end appearance skull, thalassemic facies, bone marrow expansion anemia, ineffective erythropoiesis, blood transfusion dependent thalassemia, iron overload thalassemia, iron chelation therapy, deferoxamine deferasirox deferiprone, splenectomy in thalassemia, stem cell transplantation thalassemia, Mediterranean anemia, Middle East thalassemia, Southeast Asia thalassemia, Africa thalassemia, pediatric thalassemia, adult thalassemia management


Request Online Consultation With Dr M Abdou
Fee: US$100
Secure payment via PayPal (credit and debit cards accepted)
Pay Now



Sir, Minor thalassemia patients are eligible to take corona vaccination or not?
Hi Charles,
Yes, you can go ahead with your COVID-19 vaccination as scheduled by your GP.
BW,
I have thalassemia beta minor and am 26 weeks pregnant and am unable to increase my hemoglobin levels to where they need to be and am taking floradix and hemoplex. is it even possible to increase my hemoglobin levels using iron supplements? how can i raise my hemoglobin from 8.9 to a 10?
Hi Emily,
Thank you for your comment.
The majority of patients with beta-thalassemia trait do not require any treatment, and there are no specific treatment guidelines for beta-thalassemia trait.
If there are concerns about symptomatic anaemia, serum iron and ferritin levels should be checked to assess whether iron supplementation is required or not.
Increasing your iron supplements to improve your hemoglobin is not recommended if you are not iron deficient.
Check also your serum Folate and B12 to see if you need supplements.
BW
Thank you very much. I have been overwhelmed trying to convey this to my provider who had never heard of thalassemia and i dont understand it fully myself. Also, will beta thalassemia contribute to a low platelet count?
Beta thalassemia trait (also known as beta thalassemia minor) will not contribute to, or lead to, a low platelet count.
Hi Suntarn,
Thank you for your comment.
I agree, thalassemia does not directly affect the platelet count and white blood count.
If leucocytosis occurs, it may signify an acute infection.
However, for unknown reasons, many individuals with thalassemia can have elevated neutrophil counts.
Iron deficiency anemia must be ruled out before diagnosing thalassemia.