Erythropoiesis Stimulating Medicines
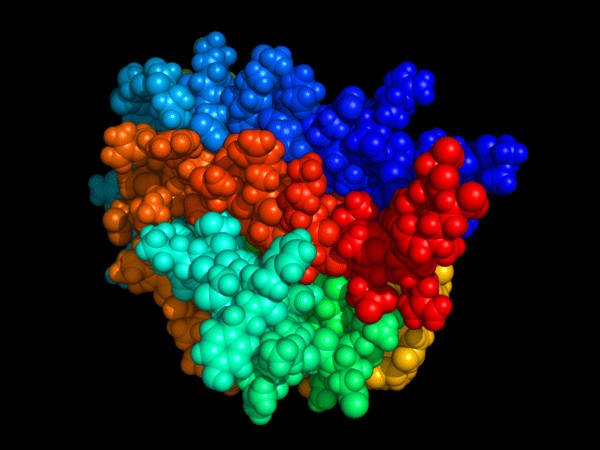
Erythropoiesis-stimulating medicines (ESAs) are synthetic forms of erythropoietin, a natural hormone produced primarily by the kidneys and, to a lesser extent, by the liver. The erythropoietin gene, located on chromosome 7 (7q21), regulates the production of red blood cells (RBCs) in the bone marrow. When oxygen levels fall, the kidney’s peritubular interstitial cells release erythropoietin, which stimulates erythroid progenitor cells to generate new RBCs and enhance the oxygen-carrying capacity of the blood. The concentration of this hormone can be measured using the erythropoietin (EPO) blood test, a useful diagnostic tool for evaluating anaemia and guiding ESA therapy.
In healthy individuals, erythropoietin secretion is finely tuned to respond to hypoxia (low blood oxygen levels). Conditions such as chronic lung disease, heart failure, or anaemia—whether from blood loss or decreased haemoglobin synthesis—trigger increased EPO production. Red blood cells contain haemoglobin, a protein that carries oxygen and gives blood its red colour. When haemoglobin or RBC levels drop, tissues receive less oxygen, leading to anaemic symptoms including fatigue, pallor, weakness, shortness of breath, and reduced exercise tolerance.
Erythropoiesis-stimulating agents such as epoetin alfa and darbepoetin alfa are commonly used to treat anaemia associated with chronic kidney disease (CKD), chemotherapy-induced anaemia, and bone marrow suppression. These agents promote erythroid activity, reduce blood transfusion requirements, and improve haemoglobin levels and quality of life. However, inappropriate ESA use or excessive haemoglobin correction can lead to thromboembolic events, hypertension, and cardiovascular complications. To ensure safety and efficacy, clinicians must adhere to current ESA dosing guidelines, perform regular haematologic monitoring, and evaluate each patient’s iron status and comorbidities before and during treatment.
Clinical indications for ESA therapy include anaemia secondary to CKD, myelodysplastic syndromes, chemotherapy, HIV therapy with zidovudine (AZT), anaemia of chronic disease, and cancer-related anaemia. ESA therapy may also be considered in low-birth-weight premature infants with anaemia. In patients with CKD, reduced renal erythropoietin output is the primary cause of anaemia, often necessitating ESA replacement.
According to U.S. FDA guidance, clinicians should consider initiating ESA therapy in patients with CKD when haemoglobin levels fall below 10 g/dL, though the precise threshold should be individualised based on clinical status, risk factors, and response to treatment. Treatment goals should focus on symptom improvement and transfusion avoidance rather than normalising haemoglobin levels.
What is recombinant erythropoietin?
In cases where transfusions are not an option—for example, when the patient cannot have or refuses, a transfusion—it may be necessary to give the patient recombinant erythropoietin. Recombinant erythropoietin is a man-made version of natural erythropoietin. It is produced by cloning the gene for erythropoietin.
Recombinant erythropoietin drugs are known as erythropoietin-stimulating agents (ESAs). These drugs are given by injection and work by stimulating the production of more red blood cells. These cells are then released from the bone marrow into the bloodstream.
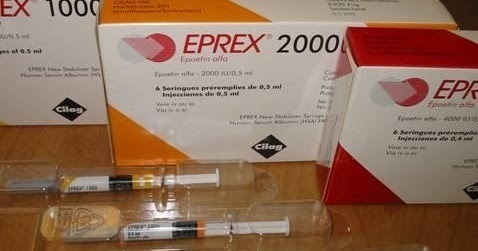
There are two ESAs on the U.S. market: epoetin alfa (Procrit®, Epogen®, Eprex®), and darbepoetin alfa (Aranesp®).
What are the side effects of ESAs?
The side effects that occur most often with ESA use include:
- High blood pressure
- Fever
- Dizziness
- Nausea
- Pain at the site of the injection.
Blood pressure should always be closely monitored in patients administered with such agents.
ESA resistance:
The working definition of ESA resistance is the requirement for greater than 150 units/kg of ESA at least 3 times per week or the sudden response refractoriness to a previous stable maintenance dose, such that hemoglobin levels fall below target levels.
The most common cause of ESA resistance is iron deficiency. Therefore, it is imperative that iron stores are adequate during ESA treatment. The second most common cause of ESA resistance is a chronic infection/inflammatory state, and such resistance is attributed to inflammatory cytokines (eg, IL-1).
Other less common causes of ESA resistance include hyperparathyroidism (the mechanism appears to be related to bone marrow fibrosis), as well as severe malnutrition.
Safety issues:
There are several safety issues with erythropoiesis-stimulating medicines:
- ESAs increase the risk of venous thromboembolism (blood clots in the veins). A blood clot can break away from one location and travel to the lung (pulmonary embolism), where it can block circulation. Symptoms of blood clots include chest pain, shortness of breath, pain in the legs, and sudden numbness or weakness in the face, arm, or leg.
- ESAs can cause hemoglobin to rise too high, which puts the patient at higher risk for heart attack, stroke, heart failure, and death.
- In patients who have cancer, ESAs may cause the tumor to grow. If ESAs are used for these patients, they are usually stopped after the patient’s chemotherapy is finished.
- The health care provider will keep an eye on the patient’s blood cell counts to make sure they do not put him or her at a higher risk. The dosing may change, depending on the patient’s needs.
Patients who have the following conditions need to consult with their health care provider if erythropoiesis-stimulating medicines are being considered as part of the treatment plan:
- Heart disease
- Uncontrolled high blood pressure
- Porphyria (a group of diseases that are caused by enzyme deficiencies)
- Seizures
- An allergy to epoetin alfa or any other part of this medicine
In addition, women who are pregnant, planning to become pregnant, or breastfeeding should consult with their health care provider before taking an ESA.
References:
Siamak N. Nabili, MD, MPH. Erythropoietin (EPO, The EPO Test). https://www.medicinenet.com/erythropoietin/article.htm#erythropoietin_epo_definition_and_facts
Erythropoietin-Stimulating Agents. Cleveland Clinic https://my.clevelandclinic.org/health/drugs/14573-erythropoietin-stimulating-agents
National Kidney Foundation. Anemia and Chronic Kidney Disease. Accessed 6/15/2018.
Edgar V Lerma, MD. Anemia of Chronic Disease and Renal Failure: Overview, Mechanism of Anemia of Chronic Disease, Prevalence of Anemia of Chronic Disease and CKD https://emedicine.medscape.com/article/1389854-overview
FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. U.S. Food & Drug Administration. Available at https://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. June 24, 2011; Accessed: November 25, 2018.
National Kidney Foundation (NKF). Clinical Practice Guideline for Anemia in Chronic Kidney Disease: 2025 Update. Available at: https://www.kidney.org/professionals/guidelines
U.S. Food and Drug Administration (FDA). Erythropoiesis-Stimulating Agents (ESAs): Drug Safety Communication—Modified Dosing Recommendations to Improve Safe Use. Updated 2023. Available at: https://www.fda.gov/drugs/drug-safety-and-availability
KDIGO (Kidney Disease: Improving Global Outcomes). Clinical Practice Guideline for Anemia in CKD. Kidney Int Suppl. 2021;11(1):1-74.
Keywords:
erythropoiesis-stimulating medicines, erythropoiesis-stimulating agents, ESAs, erythropoietin, erythropoietin hormone, erythropoietin test, EPO blood test, epoetin alfa, darbepoetin alfa, erythropoiesis stimulating drugs, erythropoietin therapy, ESA therapy, ESA treatment, anemia treatment, anaemia management, chronic kidney disease anemia, CKD anemia, chemotherapy-induced anemia, bone marrow suppression, myelodysplasia anemia, erythropoietin gene chromosome 7, red blood cell production, RBC formation, hemoglobin synthesis, oxygen-carrying capacity, ESA mechanism of action, ESA indications, ESA dosing guidelines, ESA risks and side effects, thromboembolic complications, cardiovascular risk, FDA ESA recommendations, KDIGO anemia guidelines, NICE anemia guidance, ESA in CKD, ESA in cancer, ESA monitoring, ESA safety, erythroid progenitor cells, iron status monitoring, anemia in oncology, anemia in renal disease, anemia of chronic disease, EPO level, erythropoietin deficiency, ESA response, Ask Hematologist, Dr Moustafa Abdou