Clinical Trials
Clinical research is medical research involving people. There are two types, clinical studies, and clinical trials.
Clinical studies (sometimes called observational studies) observe people in normal settings. Researchers gather information, group volunteers according to broad characteristics, and compare changes over time. For example, researchers may collect data through medical exams, tests, or questionnaires about a group of older adults over time to learn more about the effects of different lifestyles on cognitive health. Clinical studies may help identify new possibilities for clinical trials.
Clinical trials are research studies performed in people that are aimed at evaluating a medical, surgical, or behavioral intervention. They are the primary way that researchers find out if a new treatment, like a new drug or diet or medical device (for example, a pacemaker) is safe and effective in people. Often a clinical trial is used to learn if a new treatment is more effective and/or has less harmful side effects than the standard treatment.
Other clinical trials test ways to find a disease early, sometimes before there are symptoms. Still, others test ways to prevent a health problem. A clinical trial may also look at how to make life better for people living with a life-threatening disease or a chronic health problem. Clinical trials sometimes study the role of caregivers or support groups.
Before the U.S. Food and Drug Administration (FDA) approves a clinical trial to begin, scientists perform laboratory tests and studies in animals to test a potential therapy’s safety and efficacy. If these studies show favorable results, the FDA gives approval for the intervention to be tested in humans.
What Are the Benefits of Participating in a Clinical Trial?
Clinical trials are essential to the advancement of medicine. By participating in a trial, a patient may have access to a treatment that is new or better than the standard treatment. By participating in trials patients are also helping others who may benefit from the findings in the future. Some patients also receive free medical care or are paid for their participation.
Diseases affect everyone, but not in the same way. Therefore, it is important to have people of all races, ages, backgrounds, and genders participate so that the best way of preventing, diagnosing, or treating every kind of disease for every kind of person can be discovered.
Are Clinical Trials Safe?
Before an experimental treatment can be applied to people, it is carefully studied in the laboratory to determine its effectiveness and safety. In the USA, the clinical trials are reviewed at both the national level by the Food and Drug Administration (FDA) and at the local level by an institutional review board (IRB). In the UK, the clinical trials are reviewed by Medicines and Health Regulatory Agency (MHRA). Each hospital or clinic where a clinical trial is to take place has a review board made up of health-care professionals, patient advocates, and community leaders who review the trial for safety and fairness.
The clinical trials regulatory bodies only approve studies with a reasonable expectation:
1) That the study design (number of participants and what is being looked for) is appropriate to demonstrate the objective.
2) That the benefit bestowed on a participant by use of the drug/therapy or device will outweigh the risks introduced.
Possible participants are also carefully screened – by a thorough analysis of a patient’s medical history, physical examinations, and possibly other tests – to ensure that they are the best possible candidates for the experimental treatment. During the trial, patients are carefully monitored to track how the treatment is affecting their condition. Since participation in a clinical trial is voluntary, a patient can stop at any time for any reason.
What Should I Consider Before Participating in a Clinical Trial?
Before participating, you must be provided with an “informed consent” document explaining the risks and potential benefits of the trial. Be sure to read over this information carefully. It is important to fully understand the purpose of the trial and what to expect.
You will want to find out if the treatment will interact with any of your current medications or affect any other medical conditions you may have. You should be informed about what tests or procedures, such as biopsies or blood draws, will be performed, and you should consider your comfort level with what will be done. Also, think about whether you are prepared for any anticipated side effects, pain, or discomfort that may be involved.
Another consideration is how the trial will affect your daily life. Think about how long the study lasts, if it will fit in your work schedule and personal life, and if you can commit to it. Will the hours at the clinic require that you take time off work? How many visits will be involved?
If you do decide to participate, you will be required to sign an informed consent document, and a copy will be given to you. The document will include the contact information for someone you can call should any questions or concerns arise during the trial.
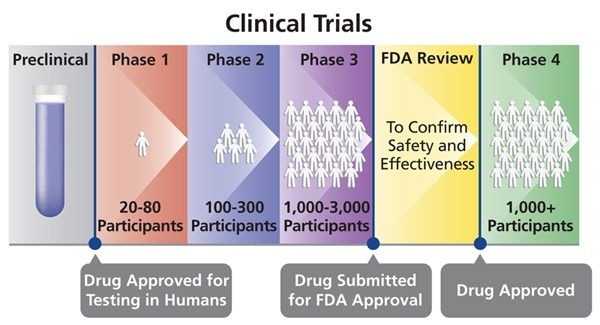
Phases of Clinical Trials:
Clinical trials advance through four phases to test a treatment, find the appropriate dosage and look for side effects. If, after the first three phases, researchers find a drug or other intervention to be safe and effective, the FDA approves it for clinical use and continues to monitor its effects.
Clinical trials of drugs are usually described based on their phase. The FDA typically requires Phase I, II, and III trials to be conducted to determine if the drug can be approved for use.
A Phase I trial tests an experimental treatment on a small group of often healthy people (20 to 80) to judge its safety and side effects and to find the correct drug dosage.
A Phase II trial uses more people (100 to 300). While the emphasis in Phase I is on safety, the emphasis in Phase II is on effectiveness. This phase aims to obtain preliminary data on whether the drug works in people who have a certain disease or condition. These trials also continue to study safety, including short-term side effects. This phase can last several years.
A Phase III trial gathers more information about safety and effectiveness, studying different populations and different dosages, using the drug in combination with other drugs. The number of subjects usually ranges from several hundred to about 3,000 people. If the FDA agrees that the trial results are positive, it will approve the experimental drug or device.
A Phase IV trial for drugs or devices takes place after the FDA approves their use. A device or drug’s effectiveness and safety are monitored in large, diverse populations. Sometimes, the side effects of a drug may not become clear until more people have taken it over a longer period of time.
Every clinical trial has a protocol or study plan that describes what will be done during the clinical trial, how the clinical trial will be conducted, and why each part of the clinical trial is necessary. The protocol or study plan also includes guidelines called eligibility criteria for who can and cannot participate in the clinical trial.
Common eligibility criteria may include:
- Having a certain type or stage of disease.
- Having received (or not having received) a certain kind of therapy in the past.
- Being in a certain age group.
- Medical history.
- Current health status.
Criteria such as these help to reduce the medical differences among people taking part in the clinical trial so that researchers can be more certain that the results are due to the treatment being tested and not to another reason.
In addition, because some people who want to take part in a clinical trial have health problems outside of the disease being studied which could be affected by the treatment being evaluated, anyone interested in joining a clinical trial will receive medical tests to confirm their eligibility.
Who performs Clinical Trials and Why?
Trials are performed to either further understanding or to provide scientific evidence to support an application for a new medicine or a new use for an existing medicine. Understanding who is leading the creation of a trial and why they are doing it is helpful.
Clinical trials involve a number of different parties. Firstly there is the “sponsor” which is the body (usually a company, university or hospital) which takes responsibility for organizing the trial and which often funds the trial. Clinical trials can be sponsored, or funded, by pharmaceutical companies, academic medical centers, voluntary groups, and other organizations. Next, there is the “investigator” who is the doctor leading the actual performance of the trial, and who is often a contractor or an employee of the sponsor. Sometimes the sponsor will engage a “CRO”, a contract research organization to help with the logistics of the performance of the trial.
Companies are commercially focused and perform trials so they can use the data they gather to support applications to government agencies that allow them to label and promote their products, on the market, for the approved application.
Government funded agencies undertake studies in the best interest of the community and to understand diseases, occasionally they will work with manufacturers or scientists to explore a particular problem, perhaps one that is not of commercial interest but is of interest to the community.
What is a randomised clinical trial?
Randomised Controlled Trials (RCTs) are one type of clinical trial. RCTs aim to find out which treatment is best by making a fair comparison between:
- a new treatment and an existing treatment.
- two (or more) existing treatments.
- a new treatment and no treatment, or a placebo (where there is no existing treatment).
RCTs – Comparing Treatment
In an RCT, two or more groups of people are compared:
- one (or more) experimental group(s) who receive a new treatment, and a control group, who receive the current standard treatment (which might be the best existing treatment, no treatment or a placebo).
- Information from the control group allows the researchers to see whether the new treatment(s) are more or less effective than the current standard treatment.
Similar groups of people
It is important that in an RCT, the two (or more) groups of people in a trial are as similar as possible, except for the treatment they receive.
This is important because it means that researchers can be sure that any differences in outcomes between the groups are only due to the treatment received.
The decision about which treatment each participant in a randomised controlled trial receives is made at random – based on chance, rather than decided by the doctor or participant. This process is called randomisation.
Randomisation
Randomisation is the best way of ensuring that the results of trials are not biased by the way participants in each group are selected.
For example, if a doctor chose which treatment a patient should receive as part of a trial, she or he might give the new treatment to sicker patients, or to younger patients. This would make the results of a trial unreliable, as it could exaggerate or hide the effects of the treatment.
Randomised controlled trials are the most reliable way to compare treatments.
Observational Studies:
Observational studies are a fundamental part of epidemiological research. They are called observational studies because the investigator observes individuals without manipulation or intervention. This is in contrast to randomised controlled trials where investigators do intervene and look at the effects of the intervention on an outcome.
For example, investigators may observe a group of older adults to learn more about the effects of different lifestyles on cardiac health.
Another example, observational studies can determine if there are associations between smoking and lung cancer, but instead of the investigator controlling who smokes or not, smoking status is observed. With appropriate control of other factors related to lung cancer and smoking status, such as gender and age, observational studies have shown a strong association between smoking and lung cancer events.
Observational studies are also useful in studying rare events where we can retrospectively collect data to determine probable causes. Additionally, observational studies help researchers know what happens in real life situations. These studies serve as a collection of data from standard practice.
In conclusion, observational studies are a key part of research. They help to investigate relationships that are unable to be tested under randomised controlled experiments, can help provide insight and develop hypotheses on what subsequent randomised evidence is needed for future research, and can provide an understanding on how things work in clinical practice.
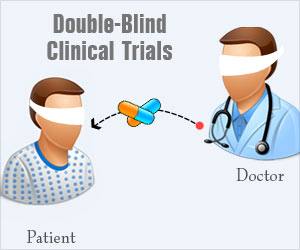
What is a Double-Blind Study?
A double-blind study is one in which neither the participants nor the experimenters know who is receiving a particular treatment. This procedure is utilized to prevent bias in research results. Double-blind studies are particularly useful for preventing bias due to demand characteristics or the placebo effect.
For example, let’s imagine that researchers are investigating the effects of a new drug. In a double-blind study, the researchers who interact with the participants would not know who was receiving the actual drug and who was receiving a placebo.
This can be contrasted with a single-blind study in which the experimenters are aware of which participants are receiving the treatment while the participants remain unaware.
In such studies, researchers may use what is known as a placebo. A placebo is an inert substance, such as a sugar pill, that has no effect on the individual taking it. The placebo pill is given to participants who are randomly assigned to the control group. A control group is a subset of participants who are not exposed to any levels of the independent variable. This group serves as a baseline to determine if exposure to the independent variable had any significant effects.
Those randomly assigned to the experimental group are given the treatment in question. Data collected from both groups is then compared to determine if the treatment had some impact on the dependent variable.
All participants in the study will take a pill, but only some of them will receive the real drug under investigation. The rest of the subjects will receive the inactive placebo. With a double-blind study, the participants and the experimenters have no idea who is receiving the real drug and who is receiving the sugar pill.
References:
Clinical Trials – American Society of Hematology. http://www.hematology.org/Patients/Trials.aspx
What Are Clinical Trials and Studies? – National Institute on Aging at NIH. https://www.nia.nih.gov/health/what-are-clinical-trials-and-studies
Clinical Trials Explained. Angelman Syndrome Ireland. http://www.angelman.ie/clinical-trials-explained/
What are clinical trials?-Novartis. https://www.novartisclinicaltrials.com/TrialConnectWeb/whatisclinicaltrials.nov
What is a randomised clinical trial? MRC Clinical Trials Unit at UCL. https://www.ctu.mrc.ac.uk/patients-public/about-clinical-trials/what-is-a-randomised-clinical-trial/
Learn About Clinical Studies. U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/about-studies/learn#ObservationalStudies
Kendra Cherry, MS. What Is a Double-Blind Study? https://www.verywellmind.com/what-is-a-double-blind-study-2795103









A beautiful piece on how a clinical trial is conducted in the clinical research industry.
Great post!! about clinical trial.
Its an informative piece on how clinical trials can benifit humans and improve their health. Thanks for sharing.
This post cells one how clinical trials give human volunteers an edge over others. Thanks for sharing.
This piece gives detailed information about clinical trials . Thanks for sharing.
I got the exact information that I needed regarding clinical trials
Nice article with a good flow and layman friendly.
Information is very helpful. Thanks for sharing
That is the right website for everyone who hopes to understand this article because you gave us excellent information about how a clinical trial is conducted in the clinical research industry.
Hi Donna,
Many thanks for your comment.
Pleased that you found this article helpful.
BW,
That is the right website for everyone who hopes to understand this article because you gave us excellent information about how a clinical trial is conducted in the clinical research industry.
Thank you so much for sharing such useful information over here with us
Hi Akash,
Thank you for your comment.
Pleased that you found this article helpful.
BW,
Thank you for taking out time and providing this informative piece of article.
Hi Kiran,
Thank you for your comment.
Pleased that you found this article helpful.
Best wishes,
Dr. M Abdou