Hairy Cell Leukemia
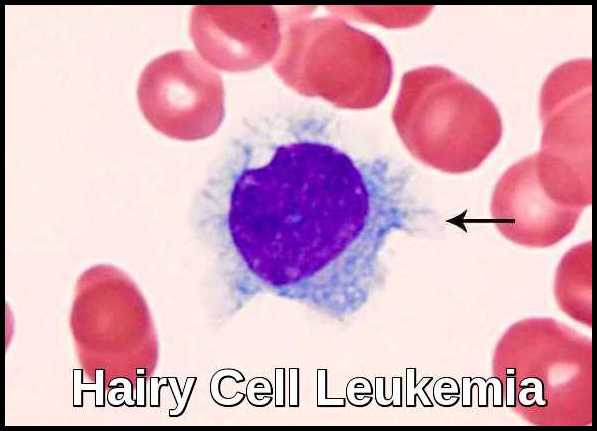
Hairy cell leukemia (HCL) is an uncommon, indolent B-cell lymphoproliferative disorder characterized by circulating lymphocytes with fine cytoplasmic projections (“hairy” appearance), splenomegaly, pancytopenia—particularly monocytopenia—and typically absent lymphadenopathy. HCL represents approximately 2% of all leukemias, with an estimated 600–800 new cases diagnosed annually in the United States and a notably low incidence in Japan. The disease occurs predominantly in white, middle-aged men, with a median age of around 52 years and a male-to-female ratio of roughly 4:1.
Clinical Picture:
The most common symptoms and presenting complaints in hairy cell leukemia are weakness and fatigue due to anemia. Approximately one-third of patients have bleeding from thrombocytopenia, and another one-third have fever and infections from neutropenia.
Symptoms related to organ infiltration of the reticuloendothelial system may occur. Abdominal discomfort from an enlarged spleen is present in one-quarter of patients.
Some patients may present with weight loss, fever, and night sweats, similar to other lymphoproliferative disorders.
Hairy cell leukemia is associated with gram-positive and gram-negative bacterial infections, as well as atypical mycobacterial and invasive fungal infections. Other opportunistic infections, such as Legionella, toxoplasmosis, and listeriosis, have been reported.
Hairy cell leukemia is associated with other systemic immunologic disorders including the following:
- Scleroderma
- Polymyositis
- Polyarteritis nodosa
- Erythematous maculopapules
- Pyoderma gangrenosum
Other uncommon conditions may be associated with hairy cell leukemia, such as acquired factor VIII antibodies, paraproteinemia, and systemic mast cell disease.
Diagnosis:

Hairy cell leukemia derives its name from the “hairy” cytoplasmic projections seen on the malignant lymphocytes (purple) under the microscope. Image credit: Internal Medicine, Nov 2016, doi: 10.2169/internalmedicine.55.6668 (CC BY-NC-ND 4.0).
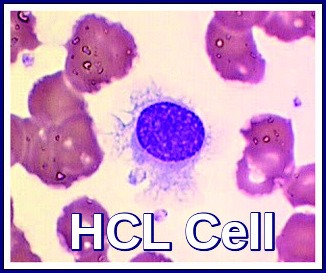
Peripheral blood smear showing a classic hairy cell leukemia lymphocyte with its distinctive “hairy” cytoplasmic projections.
In addition to the B-cell antigens CD19, CD20, and CD22, the cells coexpress CD11c, CD25, and CD103 and single or multiple immunoglobulin light chains κ λ.
The bone marrow aspirate is usually unsuccessful due to a “dry tap.” Infiltration of the bone marrow by hairy cell leukemia makes aspirating cells through a needle difficult.
Core biopsy of the bone marrow shows a pattern of hairy cell infiltration with a single round or oval nucleus separated by abundant cytoplasm in a fine fibrillar network. The cell appears separated, resulting in the characteristic fried egg appearance.
When the bone marrow aspirate is not obtainable, immunohistochemical staining of the bone marrow biopsy with either DBA.44 or anti-CD20 yields an accurate estimate of the extent of bone marrow infiltration.
In addition, it is important to recognize the occasional patients who present with hypocellular marrow and differentiate them from aplastic anemia. An accurate diagnosis is important in this highly treatable leukemia for successful patient management.
Clonal cytogenetic abnormalities are present in two-thirds of patients, and the involvement of chromosomes 1, 2, 5, 6, 11, 14, 19, and 20 have been described. Chromosome 5 abnormality is most frequent (in 40% of patients) with trisomy 5 and pericentric inversions and interstitial deletions of band 5q13.
The BRAF-V600E mutation is a hairy cell leukemia–defining genetic lesion that can be used diagnostically.
The decision to treat is based on symptomatic cytopenias, massive splenomegaly, or the presence of other complications.
About 10% of all patients will never require therapy.
Staging:
No generally accepted staging system is useful for both prognosis and therapy.
For the purpose of treatment decisions, it is best to consider this disease in the following two broad categories:
- Untreated hairy cell leukemia.
- Progressive hairy cell leukemia, either post-splenectomy or post–systemic therapy.
Untreated hairy cell leukemia
Untreated hairy cell leukemia is characterized by splenomegaly, varying degrees of leukopenia (occasionally leukocytosis) and/or pancytopenia, and bone marrow infiltration by an atypical cell with prominent cytoplasmic projections (i.e., hairy cells). The bone marrow is usually fibrotic and is not easily aspirated; therefore, bone marrow biopsies are required for diagnosis and evaluation of the degree of hairy cell infiltration.
Progressive hairy cell leukemia
Progressive hairy cell leukemia post-splenectomy (or after any systemic therapy), is characterized by progressive bone marrow replacement by hairy cells with pancytopenia refractory to treatment. For patients with advanced hairy cell leukemia treated with cladribine (2-chlorodeoxyadenosine, 2-CdA), pentostatin, or interferon-alpha, the survival rate appears to be higher than 85% at 5 years after the initiation of any one of these therapies.
Treatment:
Not all patients will require treatment immediately after the diagnosis is made and can be monitored until it is needed. This ‘watch and wait’ surveillance approach can be difficult for patients and their families and generates a lot of anxiety.
Other patients will clearly need treatment straight away because of symptoms and/or very low blood counts. The decision of when to start treatment depends upon the symptoms experienced by the patient and the degree of abnormality in the blood count.
After treatment there is almost always a further, temporary, fall in normal counts before they recover as it takes time for the disease to clear from the bone marrow. Recovery usually takes 3-6 weeks. Infection is the biggest risk, before, during and for a period after, treatment. It is important to treat any active infection effectively before any therapy for the HCL is started. It is rare that treatment is needed urgently for HCL. Therefore, careful clinical judgment is necessary to make the best decision for each individual patient regarding the optimal time to start treatment for the HCL.
For the past 30 years, the mainstay of treatment for HCL has been with 2 drugs in the group of purine analogues called pentostatin and cladribine.
Pentostatin was the first to be introduced in the 1980s and then cladribine in the early 1990s so there is now a great deal of experience and long-term follow-up with these agents. Both are highly effective at inducing a remission (in almost 100% of patients) with no significant difference between the two. Most of these remissions are complete (CR) i.e. no sign of any remaining disease in the bone marrow using standard methods. Such remissions often last for very prolonged periods of time (more than 10 years).
Both are highly effective at inducing a remission (in almost 100% of patients) with no significant difference between the two. Most of these remissions are complete (CR) i.e. no sign of any remaining disease in the bone marrow using standard methods. Such remissions often last for very prolonged periods of time (more than 10 years).
Cladribine can be given in a number of ways including as a 7-day continuous IV infusion (which may require a hospital admission), daily or weekly IV infusions for 6 doses or as a subcutaneous injection on 5 consecutive days. There is no evidence that these are not all equivalent in terms of effectiveness and the choice will largely depend on the physician and patient. Most can be given as outpatient treatment.
Pentostatin is administered as a short intravenous (IV) infusion every 2-3 weeks until a remission is achieved (usually 8-10 injections). It is excreted in the body by the kidneys and it is, therefore, important to check renal function to ensure this is normal. The dose of pentostatin needs to be reduced in renal impairment.
Both treatments are generally well tolerated but are associated with a temporary reduction in normal blood counts. This needs to be monitored closely until they recover (weekly initially). Sometimes the recovery of the blood counts can be delayed for a much longer time but eventually they do improve.
Pentostatin and cladribine also cause a more prolonged suppression of the immune system and advice should be given about the signs and symptoms of infection to be on the watch for. Infections should always be taken seriously as prompt treatment is important. Prophylactic antibiotics/antivirals e.g. Septrin and Valaciclovir are usually prescribed to reduce the risk of infections. In the case of cladribine, it is better to start these after the first-week course of treatment has been given since rashes can sometimes occur when the drugs are given concurrently. Occasionally growth factors (eg GCSF) may be given to speed the recovery of the neutrophils. Other supportive drugs such as allopurinol are not usually needed. Blood transfusions, if required, should be with irradiated blood.
Rituximab used as a single agent in first-line treatment of HCL is not as effective in inducing remissions as the purine analogues. Its use would be reserved for patients unable to tolerate purine analogues. There is early evidence that better remissions may be achieved with the combination of rituximab and a purine analogue (pentostatin or cladribine). Because of the very good results with purine analogues alone for most patients the addition of rituximab is often reserved for those patients who do not achieve a CR or who relapse earlier than expected after treatment.
Interferon is rarely used. It is not well tolerated and much less effective than the purine analogues, but occasionally may still be useful.
Splenectomy is rarely undertaken now since other therapies are so effective in reducing the size of the spleen, which is often enlarged at the time of diagnosis.
Novel Agents: Targeted therapies such as immunoconjugates (moxetumumab pasudotox), BRAF inhibitors (eg vemurafenib) and B-cell receptor inhibitors (eg ibrutinib) all have activity in HCL. Currently, these have been examined in relapsed or refractory HCL and only in a very small number of patients (in whom purine analogues cannot be given) as first-line therapy. A number of clinical trials are being undertaken to evaluate these drugs further.
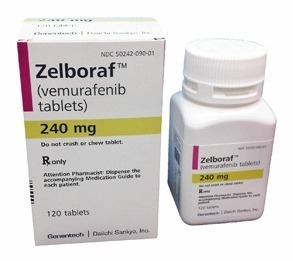
Zelboraf (vemurafenib) is a targeted BRAF-V600E inhibitor used in selected cases of refractory or relapsed hairy cell leukemia.
Clinical Trials: HCL is a rare disease and there are very few clinical studies being undertaken worldwide. It is important for patients to participate in clinical trials if at all possible, particularly if their disease has been hard to treat and they would particularly benefit from novel therapy.
Assessment of Response to Treatment and Monitoring:
Usually, within a few weeks of receiving treatment the blood counts begin to recover and will often return to completely normal levels.
However, to assess response an examination of the bone marrow is necessary. This will not be taken for at least 3-4 months as it takes time for the treatment to have its full effect.
Studies have shown that in those patients who have obtained a CR, the remission lasts longer.
There are other more sensitive laboratory methods which can assess ‘minimal residual disease’ or MRD, which may be present even in patients in a CR. It is not entirely clear what effect the presence of MRD has on outcome. MRD assessment is usually only undertaken in the setting of a clinical study and the results do not currently indicate any need to change the treatment plan.
CT or US scans may be repeated, if they were abnormal before treatment, to assess response.
Around 40% of patients will relapse, even if this is after 10 years or more. Regular monitoring will usually take place. This may only be once or twice a year for patients in a stable remission.
Relapse is usually indicated by falling blood counts and confirmed by examination of the bone marrow.
Retreatment is nearly always successful although the remissions tend to last for a shorter period of time when the same treatment is given again.
Prognosis:
The prognosis of HCL is generally favorable, with a 10-year overall survival rate of approximately 80%. However, relapse can occur in up to 40% of patients, and some patients may develop secondary malignancies.
Summary:
Hairy cell leukemia is a rare but highly treatable chronic B-cell lymphoproliferative disorder identified by its characteristic “hairy” lymphocytes on blood smear and bone marrow examination. Diagnosis is supported by immunophenotyping and confirmation of the BRAF-V600E mutation. First-line therapy typically involves purine analogues, with rituximab frequently added to deepen response and reduce relapse risk. Overall prognosis is excellent for most patients, although disease recurrence and treatment-related secondary malignancies can occasionally occur.
Questions and Answers:
What is hairy cell leukemia and how does it present?
Hairy cell leukemia is an indolent B-cell lymphoproliferative disorder that typically presents with fatigue, splenomegaly, recurrent infections, and cytopenias—especially monocytopenia and pancytopenia. Characteristic “hairy” cells are usually visible on peripheral smear or bone marrow examination.
What causes hairy cell leukemia?
Most cases of hairy cell leukemia are driven by the BRAF-V600E mutation, which leads to abnormal MAPK pathway activation and uncontrolled clonal B-cell proliferation.
How is hairy cell leukemia diagnosed?
Diagnosis combines peripheral blood smear findings, bone marrow biopsy showing trabecular fibrosis, flow cytometry (CD11c⁺, CD25⁺, CD103⁺, CD123⁺), and confirmation of the BRAF-V600E mutation. In many patients, the marrow aspirate may result in a dry tap due to fibrosis, making a core biopsy essential for confirming the diagnosis.
What are the key symptoms of hairy cell leukemia?
Common symptoms include fatigue, abdominal fullness from splenomegaly, increased infections, easy bruising, and symptoms of anemia. Many patients also have persistent monocytopenia.
What is the first-line treatment for hairy cell leukemia?
Purine analogues—particularly cladribine or pentostatin—are the standard first-line therapies and achieve high complete-remission rates.
When is rituximab used in hairy cell leukemia?
Rituximab is used for relapsed, refractory, or minimal residual disease and is often combined with purine analogues to deepen remission and reduce relapse.
Is targeted therapy available for hairy cell leukemia?
Yes. Patients with relapsed or refractory HCL can benefit from vemurafenib, a BRAF inhibitor, which is effective in BRAF-V600E–positive disease.
Can hairy cell leukemia come back after treatment?
Relapse can occur years after initial therapy. Most patients respond well to retreatment with purine analogues, rituximab, or BRAF-targeted therapy depending on prior exposure.
What is the prognosis for hairy cell leukemia?
Prognosis is generally excellent, with long-term survival in most patients following purine analogue therapy. Recurrence and secondary malignancies remain possible but manageable.
Does hairy cell leukemia require long-term follow-up?
Yes. Regular monitoring of blood counts, spleen size, and minimal residual disease is recommended to detect relapse early and guide retreatment.
References:
American Cancer Society. Hairy Cell Leukemia. Available at: https://www.cancer.org/cancer/hairy-cell-leukemia.html
Leukemia & Lymphoma Society. Hairy Cell Leukemia. Available at: https://www.lls.org/leukemia/hairy-cell-leukemia
National Cancer Institute. For Hairy Cell Leukemia, Drug Combination Leads to Long-Lasting Remissions. Available at: https://www.cancer.gov/types/leukemia/patient/hairy-cell-leukemia-treatment-pdq
Foucar K., Falini B., Catovsky D., et al. Hairy cell leukemia. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Geneva: WHO Press; 2008:188–190.
Hoffman M.A. Clinical presentations and complications of hairy cell leukemia. Hematol Oncol Clin North Am. 2006;20:1065–1073.
Jones G., Parry-Jones N., Wilkins B., et al. Revised guidelines for the diagnosis and management of hairy cell leukaemia and hairy cell leukaemia variant. Br J Haematol. 2012;156:186–195.
Gidron A., Tallman M.S. Hairy cell leukemia: towards a curative strategy. Hematol Oncol Clin North Am. 2006;20:1153–1162.
Robak T., Matutes E., Catovsky D., et al. ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up of hairy cell leukemia. Ann Oncol. 2015;26(suppl 5):v100–v107.
Dearden C.E., Matutes E., Hilditch B.L., et al. Long-term follow-up after pentostatin or cladribine in hairy cell leukemia. Br J Haematol. 1999;106:515–519.
Piro L.D., Carrera C.J., Carson D.A., et al. Lasting remissions in hairy-cell leukemia induced by a single infusion of 2-chlorodeoxyadenosine. N Engl J Med. 1990;322:1117–1121.
Cassileth P.A., Cheuvart B., Spiers A.S., et al. Pentostatin induces durable remissions in hairy cell leukemia. J Clin Oncol. 1991;9:243–246.
Thomas D.A., O’Brien S., Bueso-Ramos C., et al. Rituximab in relapsed or refractory hairy cell leukemia. Blood. 2003;102:3906–3911.
Tiacci E., Falini B. Hairy cell leukemia: a model disease for targeted therapies. Haematologica. 2018;103(4):565–574.
Grever M.R., Abdel-Wahab O., Andritsos L.A., Banerji V., Barrientos J., Blachly J.S., … Zelenetz A. Consensus guidelines for the diagnosis and management of classic hairy cell leukemia. Blood Advances. 2017;1(6):511–534.
Else M., Dearden C.E., Catovsky D. Long-term follow-up after purine analogue therapy in hairy cell leukaemia. Best Pract Res Clin Haematol. 2015;28(4):217–229.
Keywords:
hairy cell leukemia, HCL, BRAF V600E mutation, purine analogues, cladribine, pentostatin, rituximab, vemurafenib, BRAF inhibitor therapy, HCL diagnosis, HCL treatment, hairy cell leukemia symptoms, hairy cell leukemia prognosis, monocytopenia, pancytopenia, splenomegaly, lymphoproliferative disorders, bone marrow biopsy, hairy cells blood smear, flow cytometry HCL markers, CD11c CD25 CD103, targeted therapy in HCL, refractory hairy cell leukemia, relapsed hairy cell leukemia, HCL variant, classic HCL management, HCL clinical guidelines

Request Online Consultation With Dr M Abdou
Fee: US$100
Secure payment via PayPal (credit and debit cards accepted)
Pay Now


Great web site thank you , I have a question I did get treatment 2 years ago may past doing reasonably well thank god , I had a call from my doctor the other day to say my white blood cells are 4.1 and he did noticed my monocytes were a little lower than normal should I be worried ? Now this time last year my white cell count was also 4.1
My doctor said he wasn’t that concerned as all the other white cells were normal. many
Thanks Patrick
Hi Patrick,
Thank you for your comment.
White cell count is not constant and a slight reduction in your monocyte count doesn’t mean recurrence of HCL.
I understand that you had been treated, most likely with Cladribine.
If you are feeling well yourself, just repeat your CBC after 4 weeks, and if your monocyte count is persistently low or has dropped further you would need to see your Haematologist for assessment.
BW,