Gaucher’s Disease
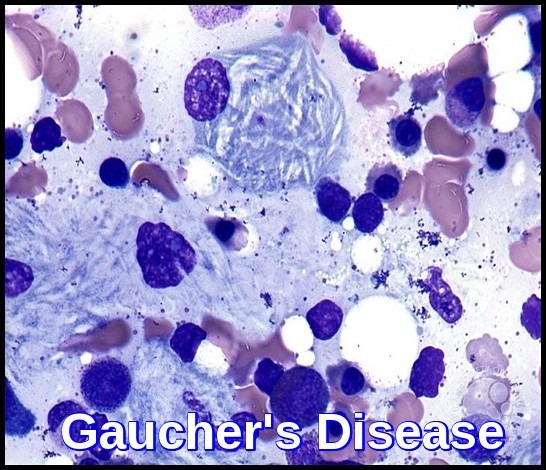
Gaucher’s disease bone marrow smear showing classic Gaucher cells with wrinkled cytoplasm (onion-skin appearance).
Gaucher’s disease is a rare inherited lysosomal storage disorder caused by deficient activity of the enzyme glucocerebrosidase. This autosomal recessive condition results from pathogenic variants in the GBA gene, leading to the accumulation of glucocerebroside within macrophages. These lipid-laden “Gaucher cells” progressively infiltrate the liver, spleen, bone marrow, and—in some subtypes—the central nervous system, producing a wide spectrum of clinical manifestations. Although Gaucher’s disease can affect individuals of any ethnicity, it is significantly more common among people of Ashkenazi Jewish descent.
The β-glucocerebrosidase deficiency causes deposition of glucocerebroside and related compounds, leading to hepatosplenomegaly, CNS changes and bony infiltration.
Glucocerebrosidase normally hydrolyzes glucocerebroside to glucose and ceramide. Genetic defects of the enzyme cause glucocerebroside accumulation in tissue macrophages through phagocytosis, forming Gaucher cells (with onion skin appearance). Accumulation of Gaucher cells in the perivascular spaces in the brain causes gliosis in the neuronopathic forms.
The course of the disease is very variable.
There are 3 types, which vary in epidemiology, enzyme activity, and manifestations:
Type I (non-neuronopathic) is the most common (90% of all patients). Residual enzyme activity is highest. Ashkenazi Jews are at greatest risk—patients with type 1 disease commonly present with painless splenomegaly, anemia, or thrombocytopenia.
Type II (acute neuronopathic) is rare, and residual enzyme activity in this type is lowest. Onset occurs during infancy. Symptoms and signs are progressive neurologic deterioration (eg, rigidity, seizures) and death by age 2 years.
Type III (subacute neuronopathic) falls between types I and II in incidence, enzyme activity, and clinical severity.
The blood film often shows features of hypersplenism and typical Gaucher cells are found in the bone marrow.
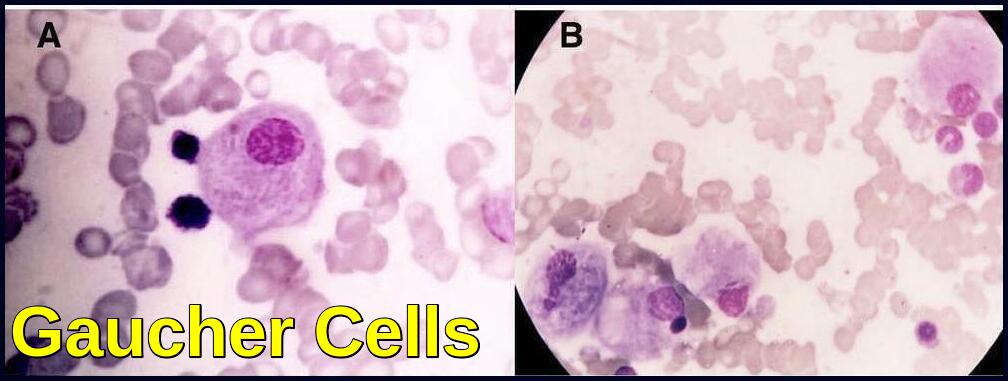
Bone marrow smear demonstrating classic Gaucher cells with enlarged cytoplasm and wrinkled, onion-skin appearance.
Clinical Manifestations:
The clinical presentation of Gaucher’s disease is highly variable, ranging from asymptomatic individuals to those with significant multi-organ involvement. Typical features include hepatosplenomegaly, anemia, thrombocytopenia, and a spectrum of skeletal complications such as osteopenia, Erlenmeyer flask deformities, bone crises, and avascular necrosis. Many patients also report fatigue, easy bruising, chronic bone pain, and an increased risk of pathological fractures.

Infographic summarizing common symptoms of Gaucher’s disease, including enlarged spleen and liver, fatigue, easy bruising, and bone pain or fractures.

X-ray demonstrating the classic Erlenmeyer flask deformity of the distal femur in Gaucher’s disease.
Diagnosis:
Accurate and timely diagnosis of Gaucher’s Disease is essential for initiating appropriate management. Hematologists often utilize a combination of clinical findings, imaging studies, and laboratory tests. The demonstration of Gaucher cells in bone marrow aspirates or biopsies is a definitive diagnostic method. Molecular genetic testing to identify GBA gene mutations further supports the diagnosis.
The diagnosis can be confirmed through measurement of glucocerebrosidase activity in WBCs. A finding of less than 15% of mean normal activity is diagnostic; carriers are detected, and types are distinguished by mutation analysis.
Case Report:
A 6-year-old boy presented with splenomegaly 15 cm below the left costal margin with a history of anemia since the age of 8 months. The liver was palpable to approximately 1 cm below the right costal margin. Abdominal ultrasound confirmed marked splenomegaly and hepatomegaly. Complete blood counts revealed pancytopenia. WBC count was: 2.7 x 10^9/L, Hemoglobin was : 10.0 g/dL, PLT: 98 x 10^9/L. A bone marrow biopsy was performed due to the patient’s persistent pancytopenia and splenomegaly. The bone marrow aspirate showed normal erythroid and myeloid maturation. Megakaryocytes were present in normal numbers. Individual as well as large clusters and aggregates of macrophages with eccentric nuclei, abundant blue-gray cytoplasm and wrinkled tissue paper appearance are seen.
Enzymatic quantification of β-glucocerebrosidase was performed which confirmed it’s deficit.
Gaucher’s Disease ( GD) is usually diagnosed by the demonstration of characteristic “Gaucher cells” in the bone marrow. Pseudo-Gaucher cells have occasionally been described in various hematologic malignancies including multiple myeloma, myelodysplastic syndrome, lymphomas, chronic myelogenous leukemia and thalassemia.
Therefore, detection of reduced or absent β-glucosidase (glucocerebrosidase) enzyme activity is the gold standard for the diagnosis of all the variants of GD.
Treatment:
Enzyme replacement therapy (ERT) has revolutionized the management of Gaucher’s Disease. Recombinant glucocerebrosidase is administered intravenously, effectively reducing the accumulation of glucocerebroside and ameliorating symptoms. Substrate reduction therapy (SRT) and, in severe cases, hematopoietic stem cell transplantation (HSCT) may also be considered.
Treatment for types I and III include enzyme replacement with glucocerebrosidase, and sometimes miglustat, eliglustat, splenectomy, or stem cell or bone marrow transplantation; there is no treatment for type II.
The 3 FDA-approved drugs available for treating Gaucher disease include:
Cerezyme® (imiglucerase)
ELELYSO® (taliglucerase alfa)
VPRIV® (velaglucerase alfa)
N.B.-specific enzyme replacement therapy is very expensive!
Summary:
Gaucher’s disease is a rare autosomal recessive lysosomal storage disorder caused by pathogenic GBA gene mutations leading to deficient glucocerebrosidase activity. As a result, lipid-laden Gaucher cells accumulate within the liver, spleen, bone marrow, and—in some types—the central nervous system. Clinical features range from asymptomatic to severe multisystem disease, with common manifestations including hepatosplenomegaly, anemia, thrombocytopenia, fatigue, easy bruising, bone crises, osteopenia, avascular necrosis, and the characteristic Erlenmeyer flask deformity. Diagnosis relies on low glucocerebrosidase enzyme levels, GBA mutation testing, and supportive findings on bone marrow examination. Current treatment options include enzyme replacement therapy (ERT), substrate reduction therapy (SRT), and supportive management for skeletal and hematologic complications. Early recognition and targeted therapy significantly improve long-term outcomes.
Questions and Answers:
What is the main cause of Gaucher’s disease?
Gaucher’s disease is caused by pathogenic mutations in the GBA gene, which lead to deficient glucocerebrosidase enzyme activity and the accumulation of glucocerebroside inside macrophages, forming Gaucher cells.
What are the typical symptoms of Gaucher’s disease?
Common symptoms include hepatosplenomegaly, anemia, thrombocytopenia, fatigue, easy bruising, bone pain, bone crises, osteopenia, and the characteristic Erlenmeyer flask deformity seen on imaging.
What do Gaucher cells look like under the microscope?
Gaucher cells are enlarged macrophages with abundant pale cytoplasm that appears wrinkled or “onion-skin” in texture, commonly found in the bone marrow, spleen, and liver.
What is the Erlenmeyer flask deformity in Gaucher’s disease?
The Erlenmeyer flask deformity refers to the flared, widened appearance of the distal femur or proximal tibia on X-ray, caused by defective bone remodeling in Gaucher’s disease.
How is Gaucher’s disease diagnosed?
Diagnosis is based on markedly reduced glucocerebrosidase enzyme activity, confirmation through GBA gene mutation testing, supportive bone marrow findings, and assessment of organ involvement.
Can Gaucher’s disease affect the bones?
Yes. Gaucher’s disease commonly causes bone crises, chronic bone pain, osteopenia, avascular necrosis, pathological fractures, and the classic Erlenmeyer flask deformity.
What treatments are available for Gaucher’s disease?
The main treatments include enzyme replacement therapy (ERT) to restore enzyme activity, substrate reduction therapy (SRT) to decrease lipid accumulation, and supportive care for hematological and skeletal complications.
Is Gaucher’s disease more common in certain populations?
Gaucher’s disease can occur in any ethnicity, but Type 1 is significantly more common among individuals of Ashkenazi Jewish descent due to a higher carrier frequency of specific GBA mutations.
Can children develop Gaucher’s disease?
Yes. Pediatric patients may present with early splenomegaly, anemia, growth delays, bone deformities, or neurological involvement depending on the disease subtype.
What imaging abnormalities are seen in Gaucher’s disease?
Radiologic findings include the Erlenmeyer flask deformity, osteopenia, lytic lesions, bone infarctions, and avascular necrosis, reflecting impaired bone remodeling.
Does Gaucher’s disease affect the nervous system?
Neurological involvement occurs in Types 2 and 3 and may include seizures, developmental delay, oculomotor abnormalities, and progressive neurodegeneration.
References:
Mistry PK, Taddei T, vom Dahl S, Rosenbloom BE. Gaucher disease and malignancy: a model for cancer pathogenesis in an inborn error of metabolism. Crit Rev Oncog. 2013;18(3):235–246.
Zimran A, Belmatoug N, Bembi B, et al. Demographics and patient characteristics of 1209 patients with Gaucher disease: descriptive analysis from the Gaucher Outcome Survey (GOS). Am J Hematol. 2018;93(2):205–212.
Wexler, Marisa, MS. Gaucher disease symptoms. Updated April 7, 2025. Fact-checked by Inês Martins, PhD. Gaucher Disease News. Available at: https://gaucherdiseasenews.com/symptoms/
Rupali Parikh, MD. Gaucher’s cells in bone marrow aspirate smears. ASH Image Bank. American Society of Hematology. Available at: https://imagebank.hematology.org/image/62962/gauchers-cells-in-bone-marrow-aspirate-smears
ResearchGate Authors. Radiograph showing Erlenmeyer flask deformity in Gaucher disease. In: Lysosomal storage diseases – Scientific Figure on ResearchGate. Available from:
https://www.researchgate.net/figure/Gaucher-disease-Radiograph-of-lower-extremities-showing-Erlenmeyer-flask-deformity-of_fig13_308855498
Keywords:
Gaucher’s disease, GBA gene mutation, glucocerebrosidase deficiency, Gaucher cells, lysosomal storage disorder, hepatosplenomegaly, thrombocytopenia, anemia, bone crises, Erlenmeyer flask deformity, bone marrow Gaucher cells, lipid-laden macrophages, enzyme replacement therapy, substrate reduction therapy, type 1 Gaucher disease, type 2 Gaucher disease, type 3 Gaucher disease, Gaucher disease symptoms, Gaucher disease diagnosis, Gaucher disease treatment, skeletal complications in Gaucher disease, bone marrow infiltration, avascular necrosis, bone pain Gaucher, Ashkenazi Jewish Gaucher disease, GBA mutation testing, glucocerebroside accumulation, radiographic features Gaucher disease, lysosomal storage diseases, bone deformity Gaucher, hematologic complications Gaucher disease


Request Online Consultation With Dr M Abdou
Fee: US$100
Secure payment via PayPal (credit and debit cards accepted)
Pay Now